Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Endpoints assessed at the population or community level are most often the result of the sum of effects on individuals, arising from the effects at the cellular and molecular levels. Within this framework, these lower biological level endpoints are more responsive at an early stage of exposure, making them potential toolboxes to be used as early-warning markers to address stress. Given this, by linking responses and understanding organisms’ metabolism and physiology, the possibilities for the use of biomarkers in stress biology are vast.
- cost of tolerance
- ecotoxicology
- energetics
- integrated biomarker response
- mechanisms of action
- neurotoxicity
- oxidative stress
1. Introduction to Stress Biology and Biomarkers
Since the early days of mankind and sky rising after the industrial revolution, freshwater, marine, and terrestrial ecosystems have been subjected to a myriad of contaminants and consequent impacts, prompting the need for the establishment of pollution control regulations and monitoring programs. First focused on chemical and microbiological analysis, it was clear that these alone were not sufficient to monitor and protect aquatic ecosystems, and thus the need to develop tools capable of assessing not only the presence of pollutants in the water, but also, and most of all, their latent effects on organisms, their populations, and communities was paramount [1]. To contribute to the environmental health assessments and eventual impact of pollutants, assemblages and diversity of fish, invertebrates, algae, and macrophytes have been used as bioindicators of pollution and water quality for several years to date [2][3][4][5] and are important elements of government regulations and ecological risk assessment (ERA) (e.g., [6][7]).
By using these high levels of biological organization, this type of assessment is therefore highly robust and relevant, and easy to apply and interpret. Using them, it is possible to evaluate the direct effects of anthropogenic or natural disturbances on the structure and/or function of an actual community of aquatic organisms, which cannot be observed when using lower levels of biological organization [8][9]. However, by addressing populations and communities, the specific causes of the observed effects may not be assessed. These, being longer-term effects, may include a temporal gap between the cause and effects that may be difficult to interpret. Also, the predictive capacity of those measurements is restricted because repeatability is difficult, and they may not clearly distinguish between a polluted site and a naturally impoverished one. Despite the relevance of such a level of organization assessment, the major drawback with this approach is that ecologically important effects such as impairment in growth, behaviour, reproduction, death, or even taxa losses, will have already occurred prior to being detected at the population and community levels [10].
It is in this framework that modern ecotoxicology thrives, as the scientific discipline combining methods of ecology and toxicology to study the effects of environmental stressors, i.e., environmental conditions deviating from species optima, while the relevance of responses is paramount to strengthen this field.
2. Linking Levels of Biological Organization
Effects assessed at higher levels of biological organization (populations and communities) are most often the result of the sum of effects on individuals, arising from the effects at the cellular and molecular levels [9]. Given this rationale, these lower biological organization levels are more responsive at an early stage, allowing them to be used as early warning endpoints to address environmental stress.
This way, the information concerning impacts at the molecular level of biological organisation (e.g., transcripts or proteins) may allow for an early-in-time assessment of future ecosystem problems, which will eventually enable for a timely intervention, before the impacts are visible and irreversible.
However, despite providing an early warning and an increased knowledge of the toxicity mechanisms, allowing the protection of biological integrity, the major setback is that these endpoints may fail to foresee later impacts on the environment, due to ecosystem resilience or weak link to the effects in the following level of biological organization, making these tools just too conservative for stakeholder interests [11]. Hence, an approach to targeting lower levels will always require addressing the potential effects at higher levels of biological organization by establishing a link of biological organization where the effects assessed at the lower end of the biological organization axis (if of sufficient duration and magnitude) are linked, with a high probability of causing effects, to the other end, including to populations and communities, and eventually causing ecosystem alterations later in time [9] (Figure 1).
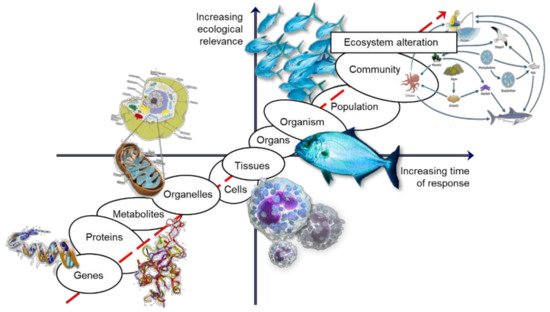
Figure 1. The biological organization continuum, relationship between temporal scale of response and ecological relevance after stress exposure (adapted from Lemos et al. 2010 [9]).
Within this framework, biomarkers arise as a resourceful sub-individual tool in ecotoxicology. As with many other techniques applied in environmental sciences, arising from health science as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [12] or a “chemical, its metabolite, or the product of an interaction between a chemical and some target molecule or cell that is measured in the human body” [13], biomarkers are presently well established to address the biological effects of environmental contamination.
Similar to everyday use of body temperature, used as a proxy for fever, or even cholesterol that is acknowledged as a biomarker of cardiovascular risk, many are the endpoints that may be considered in other disciplines other than public health, including environmental sciences, where the use of biomarkers was initially promoted in the 1990s.
The environmental biomarkers can then be defined as “any biological response to an environmental chemical at the below individual level, measured inside an organism or in its products (urine, faeces, hairs, feathers, etc.), indicating a departure from the normal status, that cannot be detected from the intact organism” [14], or following Depledge [15] defined as a “biochemical, cellular, physiological or behavioural variation that can be measured in tissue or body fluid samples or at the level of whole organisms, to provide evidence of exposure and/or effects from one or more contaminants”. Both definitions, for modern purposes, should replace “chemical agents” by “stressor” to include other biotic and abiotic features that impact organisms. They are used to characterize stressors’ mode of action, to establish cause–effect relationships, to point to the presence of a certain group of contaminants, and for environmental health monitoring [16]. As already identified, in environmental studies and decision-making, the use of these endpoints is frequently critiqued for their limited ecological relevance as of unknown ecological significance of many sub-cellular responses [9]. This means that a sole significant response of a sub-individual endpoint may prove to be meaningless (or of limited scope, solely indicating exposure) and the cornerstone challenge is to find relationships between these biomarkers and ecologically relevant parameters, and thus obtain the full power of biomarkers as endpoints with high probability to be early warning signals of what may happen in the future to the population/community, while also enabling us to dig deep into a stressor impact mechanistic understanding.
One of the most comprehensive examples includes the biomarker acetylcholinesterase (AChE; EC 3.1.1.7). This may be included in a group of neurotoxicity biomarkers which may give a sign of neuromuscular disorder when addressing the acetylcholine receptor-ion channel complex (AChR) of skeletal muscles. Briefly, the nervous impulse passes when the molecule acetylcholine travels through the axons from the presynaptic membrane to the postsynaptic membrane. At the postsynaptic membrane it connects to a receptor opening an ion channel and the nervous impulse passes through. When the enzyme acetylcholinesterase catabolizes acetylcholine in choline and acetate, the receptor-acetylcholine complex is broken and the nervous impulse stops. Thus, AChE acts as a key of this nervous impulse and has a paramount role as a neuromuscular mediator. As a case study on the use of this biomarker, one may recall to the work of Venkateswara Rao et al. [17] addressing the effects of organophosphates in the brine shrimp, Artemia salina. In this study, Chloropyrifos, Profenofos, Monocrotophos, and Acephate were used as exposure media in the laboratory, and AChE activity was evaluated. For all tested organophosphates, there was a significant inhibition of AChE. Notwithstanding, despite this activity inhibition, one can argue about what does it mean and what it might represent in the biomarker world and shout a “so what?”—as stated before, the low relevance of this level is generally weakly linked to any meaningful real ecological impact. To establish a probabilistic relation with relevant scenarios, this study’s authors monitored the brine shrimp’s behaviour in an arena through video-tracking, where endpoints such as distance travelled, and speed were recorded after exposure to the organophosphates. Authors found a significant reduction in distance travelled and speed in those brine shrimp exposed to the compounds. By linking the AChE inhibition to impaired behaviour, one may now argue about the usefulness of this biomarker, as this link through these levels of biological organization may imply that enzyme inhibition may lead to behavioural impairment and when an organism moves in an increasingly less coordinated way, it will diminish the chances to find food or escape predators (e.g.,) and thus having impacts on survival, growth or reproduction and thus the implicated effects on population numbers and dynamics.
With this example, relevance to a low level of biological organization is given by linking probabilistically to higher levels of biological organization, while maintaining the backbone of the biomarkers’ advantage; that is, to give earlier responses while providing a mechanistic overview of the effects.
The aforementioned example is transversal to a myriad of environments and taxa, with many other authors also establishing such model links, such as with the bivalve Corbicula fluminea, exposed to the pyrethroid insecticide bifenthrin [18], or the common prawn Palaemon serratus exposed to deltamethrin [19], or the freshwater Daphnia magna exposed to chlorpyrifos [20], or the coho salmon Oncorhynchus kisutch exposed to chlorpyrifos [21], or even in the terrestrial environment the earthworm Eisenia fetida exposed to atrazine [22], or the carabid beetle Pterostichus cupreus [23], or the common shrew Sorex araneus [24], exposed to dimethoate, just to give few examples and all linking AChE to behaviour and establishing consequent causal effects at higher levels.
Still, much can be discussed and studied about the degree to which a given impact at a certain level will increase the chance of having effects also at the following level—and this is paradigmatic for biomarkers, as a whole, and definitely one of the biggest challenges for its use for regulatory purposes.
This entry is adapted from the peer-reviewed paper 10.3390/biology10121340
References
- Lam, P.K. Use of biomarkers in environmental monitoring. Ocean Coast. Manag. 2009, 52, 348–354.
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27.
- Small, A.M.; Adey, W.H.; Lutz, S.M.; Reese, E.G.; Roberts, D.L. A macrophyte-based rapid biosurvey of stream water quality: Restoration at the watershed scale. Restor. Ecol. 1996, 4, 124–145.
- Lavoie, I.; Campeau, S.; Grenier, M.; Dillon, P.J. A diatom-based index for the biological assessment of eastern Canadian rivers: An application of correspondence analysis (CA). Can. J. Fish. Aquat. Sci. 2006, 63, 1793–1811.
- Johnson, R.K.; Hering, D. Response of taxonomic groups in streams to gradients in resource and habitat characteristics. J. Appl. Ecol. 2009, 46, 175–186.
- United States Clean Water Act (PL 92–500) 1972. p. 92e500. Available online: https://www.govinfo.gov/content/pkg/STATUTE-86/pdf/STATUTE-86-Pg816.pdf (accessed on 1 November 2021).
- The EU Water Framework Directive. European parliament council directive 2000/60/EC of the european parliament and of the council of 23 October 2000, establishing a framework for community action in the field of water policy. Off. J. Eur. Union 2000, 327, 1–73.
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV Cellular energy allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55.
- Lemos, M.F.L.; Soares, A.M.V.M.; Correia, A.; Esteves, A.C. Proteins in ecotoxicology—How, why and why not? Proteomics 2010, 10, 873–887.
- Maltby, L. Studying stress: The importance of organism-level responses. Ecol. Appl. 1999, 9, 431–440.
- Lam, P.K.S.; Gray, J.S. The use of biomarkers in environmental monitoring programmes. Mar. Pollut. Bull. 2003, 46, 182–186.
- National Institutes of Health. Biomarkers definitions working group biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95.
- World Health Organization International Programme on Chemical Safety. Biomarkers and Risk Assessment: Concepts and Principles/Published under the Joint Sponsorship of the United Nations Environment Programme; The International Labour Organisation; World Health Organization: Geneva, Switzerland, 1993.
- Van Gestel, C.A.M.; van Brummelen, T.C. Incorporation of the biomarker concept in ecotoxicology calls for a redefinition of terms. Ecotoxicology 1996, 5, 217–225.
- Depledge, M.H. The rational basis for the use of biomarkers as ecotoxicological tools. In Nondestructive Biomarkers in Vertebrates; Fossi, M.C., Leonzio, C., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 261–285.
- Adams, S.M.; Giesy, J.P.; Tremblay, L.A.; Eason, C.T. The use of biomarkers in ecological risk assessment: Recommendations from the Christchurch conference on Biomarkers in Ecotoxicology. Biomarkers 2001, 6, 1–6.
- Venkateswara Rao, J.; Kavitha, P.; Jakka, N.M.; Sridhar, V.; Usman, P.K. Toxicity of organophosphates on morphology and locomotor behavior in brine shrimp, Artemia salina. Arch. Environ. Contam. Toxicol. 2007, 53, 227–232.
- Zhang, H.; Hong, X.; Yan, S.; Zha, J.; Qin, J. Environmentally relevant concentrations of bifenthrin induce changes in behaviour, biomarkers, histological characteristics, and the transcriptome in Corbicula fluminea. Sci. Total Environ. 2020, 7281, 138821.
- Oliveira, C.; Almeida, J.; Guilhermino, L.; Soares, A.M.V.M.; Gravato, C. Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat. Toxicol. 2012, 124, 209–216.
- Ferrario, C.; Parolini, M.; de Felice, B.; Villa, S.; Finizio, A. Linking sub-individual and supra-individual effects in Daphnia magna exposed to sub-lethal concentration of chlorpyrifos. Environ. Pollut. 2018, 235, 411–418.
- Sandahl, J.F.; Baldwin, D.H.; Jenkins, J.J.; Scholz, N.L. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 2005, 24, 136–145.
- Lammertyn, S.; Masín, C.E.; Zalazar, C.S.; Fernandez, M.E. Biomarkers response and population biological parameters in the earthworm Eisenia fetida after short term exposure to atrazine herbicide. Ecol. Indic. 2021, 121, 107173.
- Jensen, C.S.; Garsdal, L.; Baatrup, E. Acetylcholinesterase inhibition and altered locomotor behavior in the carabid beetle Pterostichus cupreus. A linkage between biomarkers at two levels of biological complexity. Environ. Toxicol. Chem. 1997, 16, 1727–1732.
- Dell’Omo, G.; Turk, A.; Shore, R.F. Secondary poisoning in the common shrew (Sorex araneus) fed earthworms exposed to an organophosphate pesticide. Environ. Toxicol. Chem. 2009, 18, 237–240.
This entry is offline, you can click here to edit this entry!
