Skin substitutes can provide a temporary or permanent treatment option for chronic wounds. The selection of skin substitutes depends on several factors, including the type of wound and its severity. Full-thickness skin grafts (SGs) require a well-vascularised bed and sometimes will lead to contraction and scarring formation. Besides, donor sites for full-thickness skin grafts are very limited if the wound area is big, and it has been proven to have the lowest survival rate compared to thick- and thin-split thickness. Tissue engineering technology has introduced new advanced strategies since the last decades to fabricate the composite scaffold via the 3D-bioprinting approach as a tissue replacement strategy. Considering the current global donor shortage for autologous split-thickness skin graft (ASSG), skin 3D-bioprinting has emerged as a potential alternative to replace the ASSG treatment. The three-dimensional (3D)-bioprinting technique yields scaffold fabrication with the combination of biomaterials and cells to form bioinks.
- 3D-bioprinting
- cellular activity
- precision medicine
- bioinks
- wound healing
- biomaterials
1. Introduction
1.1. Wound Healing
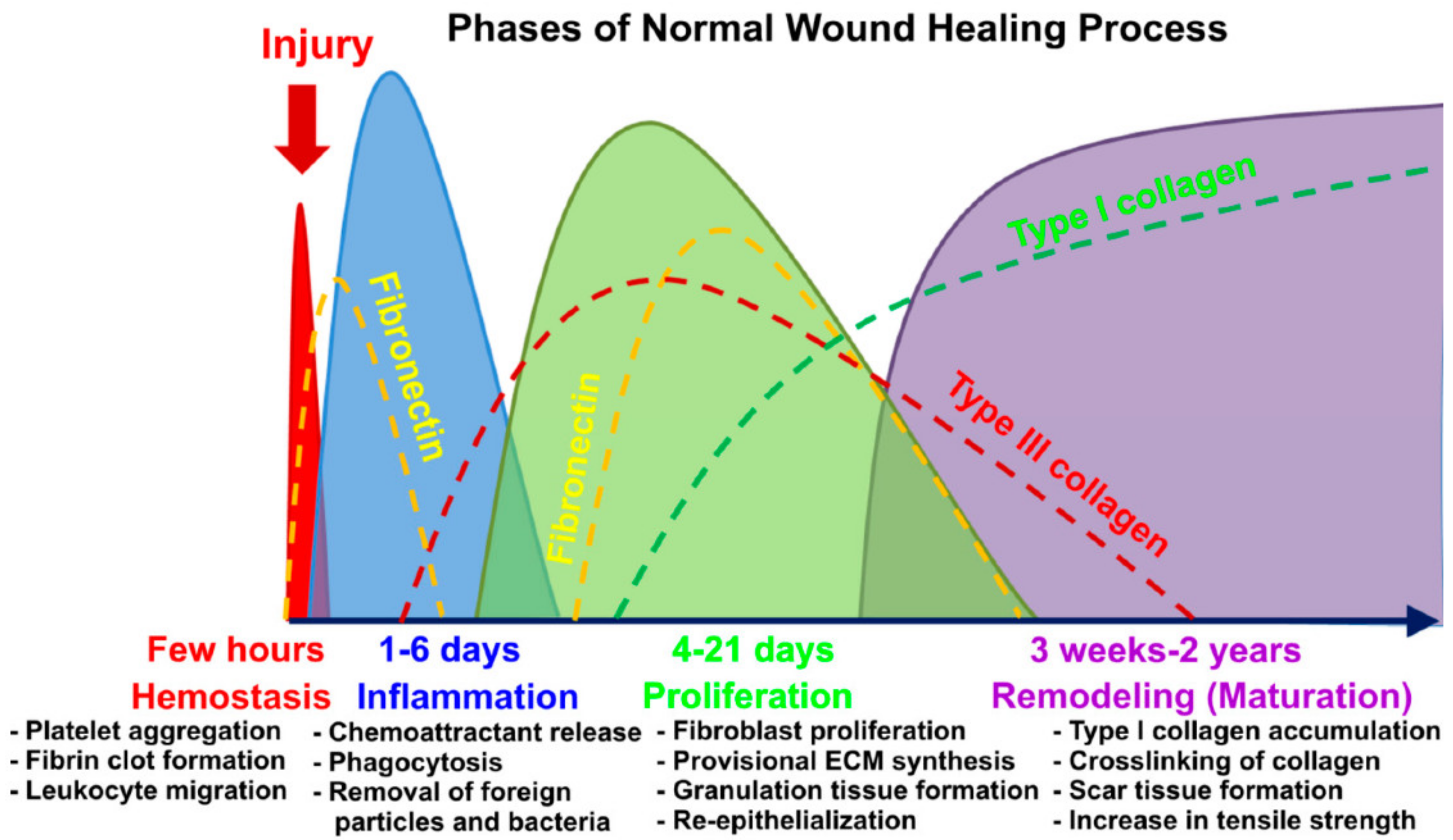
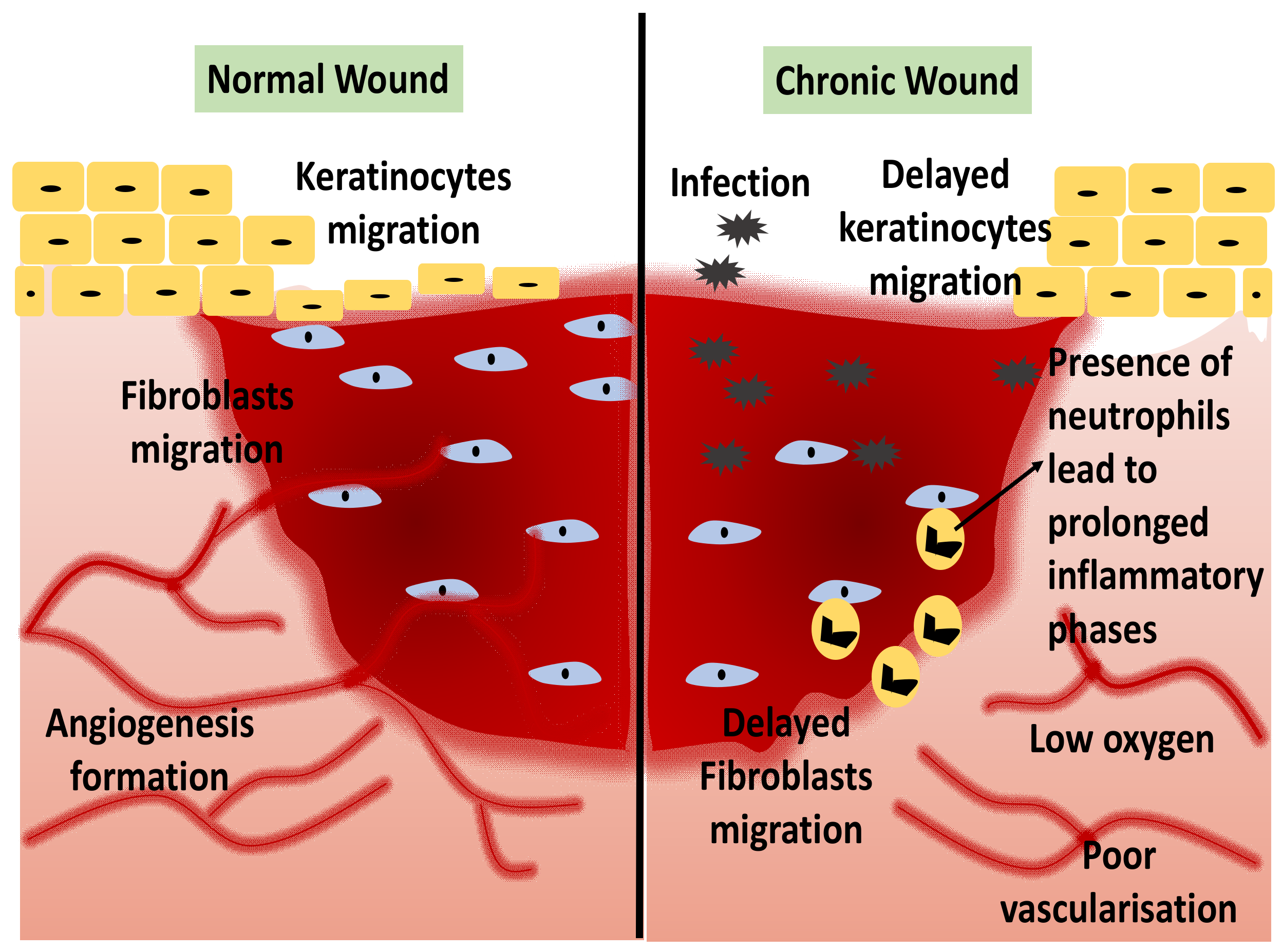
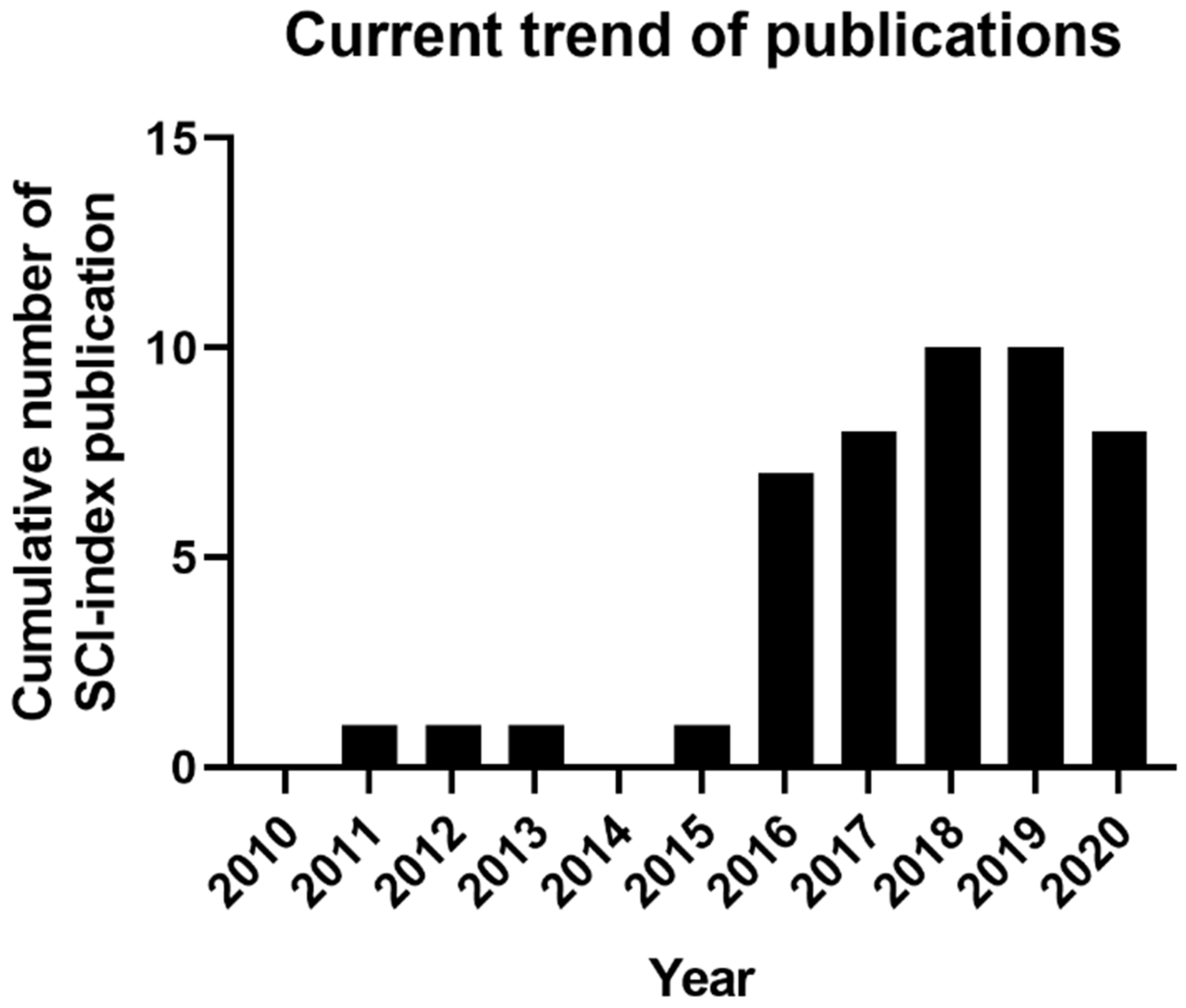
1.2. Current Trend of 3D-Bioprinting for Chronic Wound
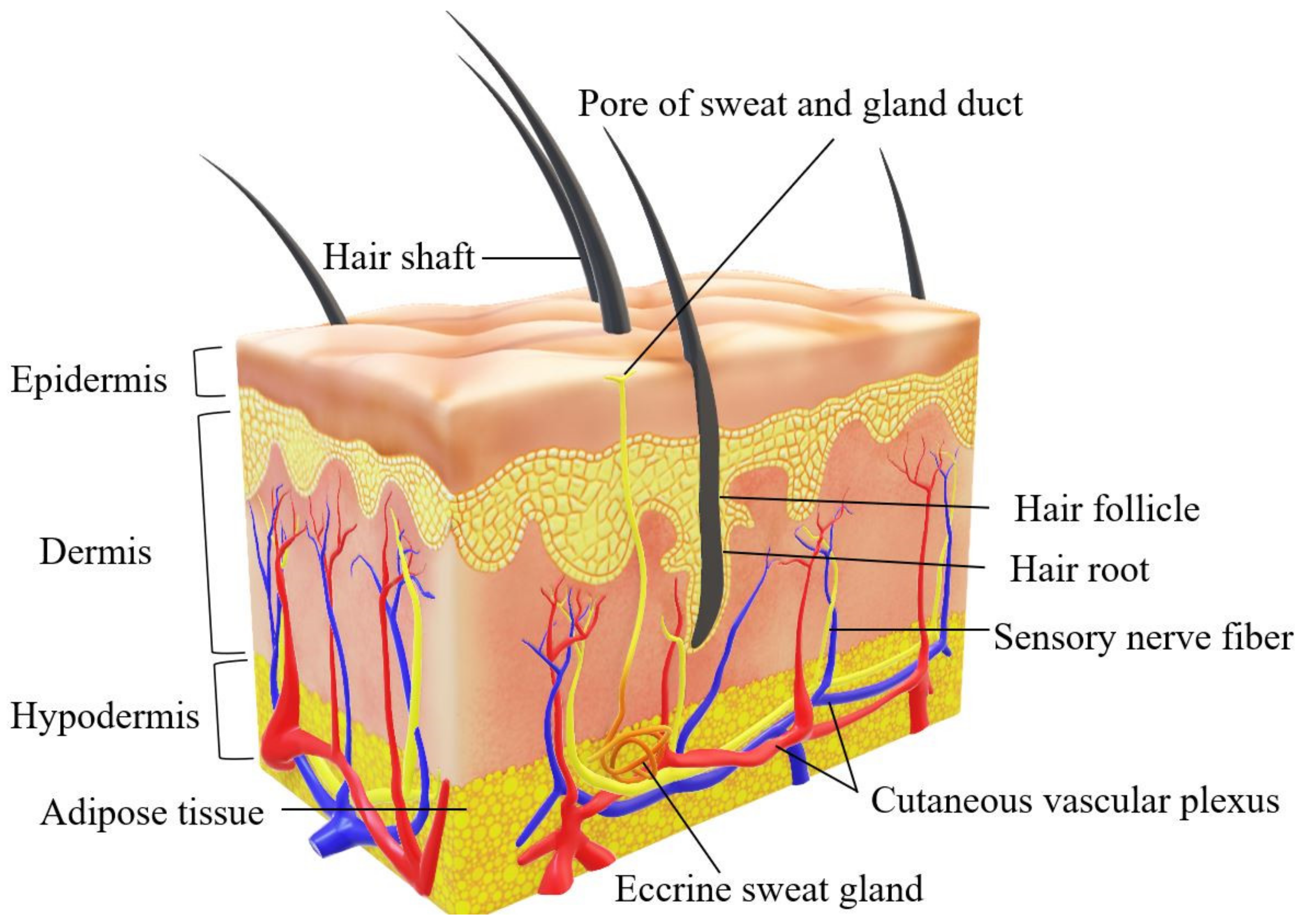
2. Human Skin Structure
3. 3D-Bioprinting for Chronic Wound
3.1. In Vitro Skin 3D-Bioprinting
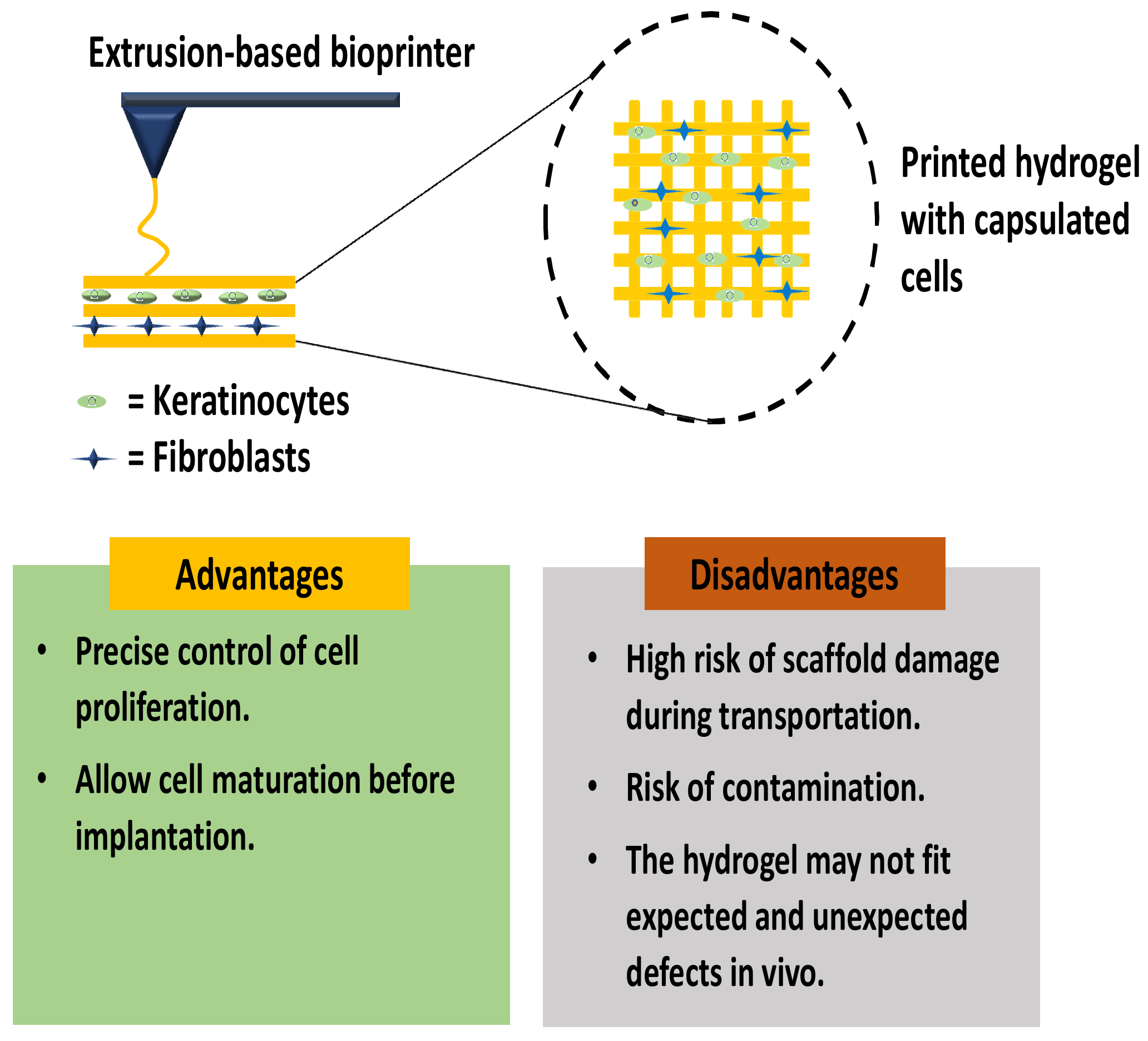
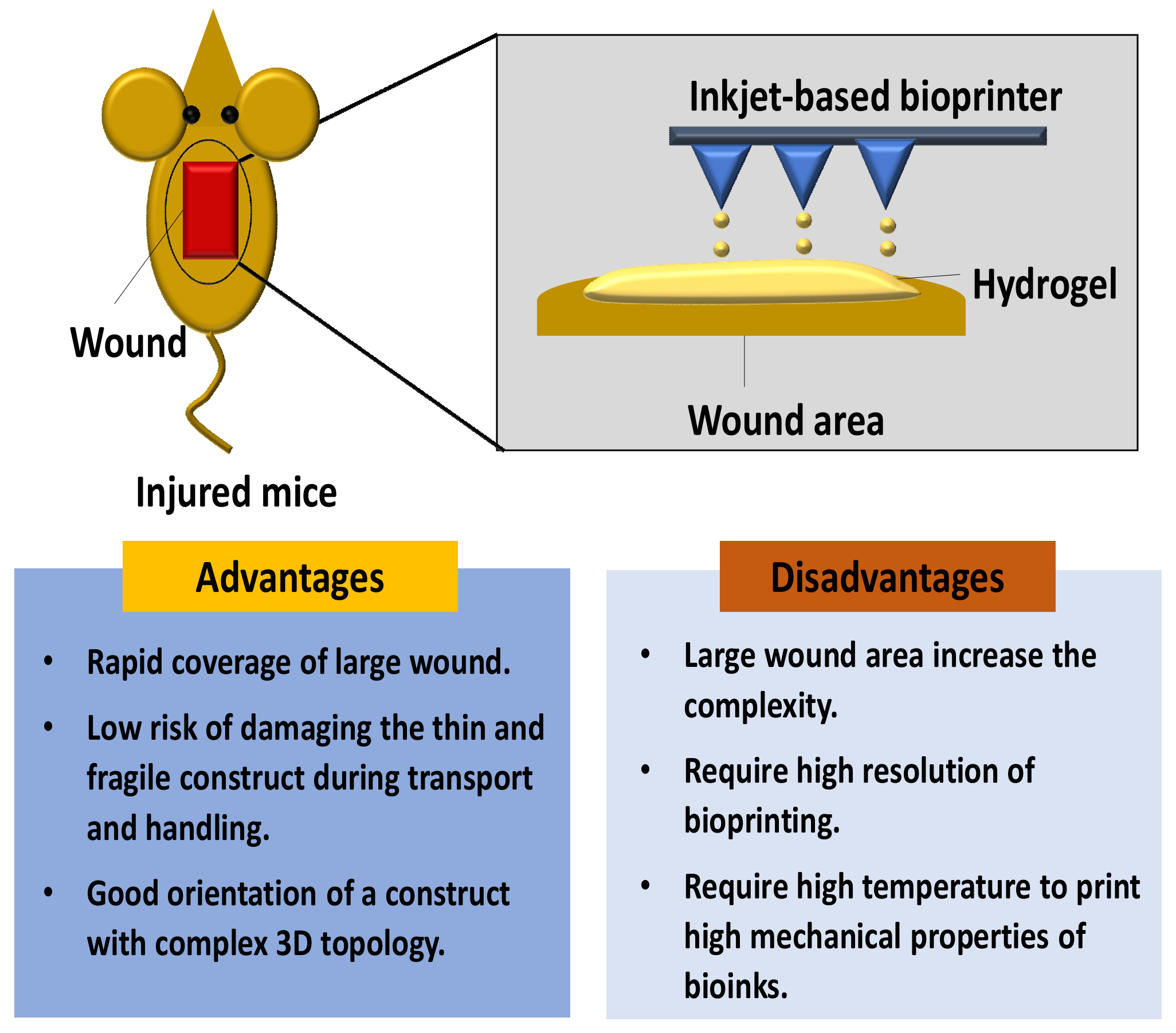
3.2. In Situ Skin 3D-Bioprinting
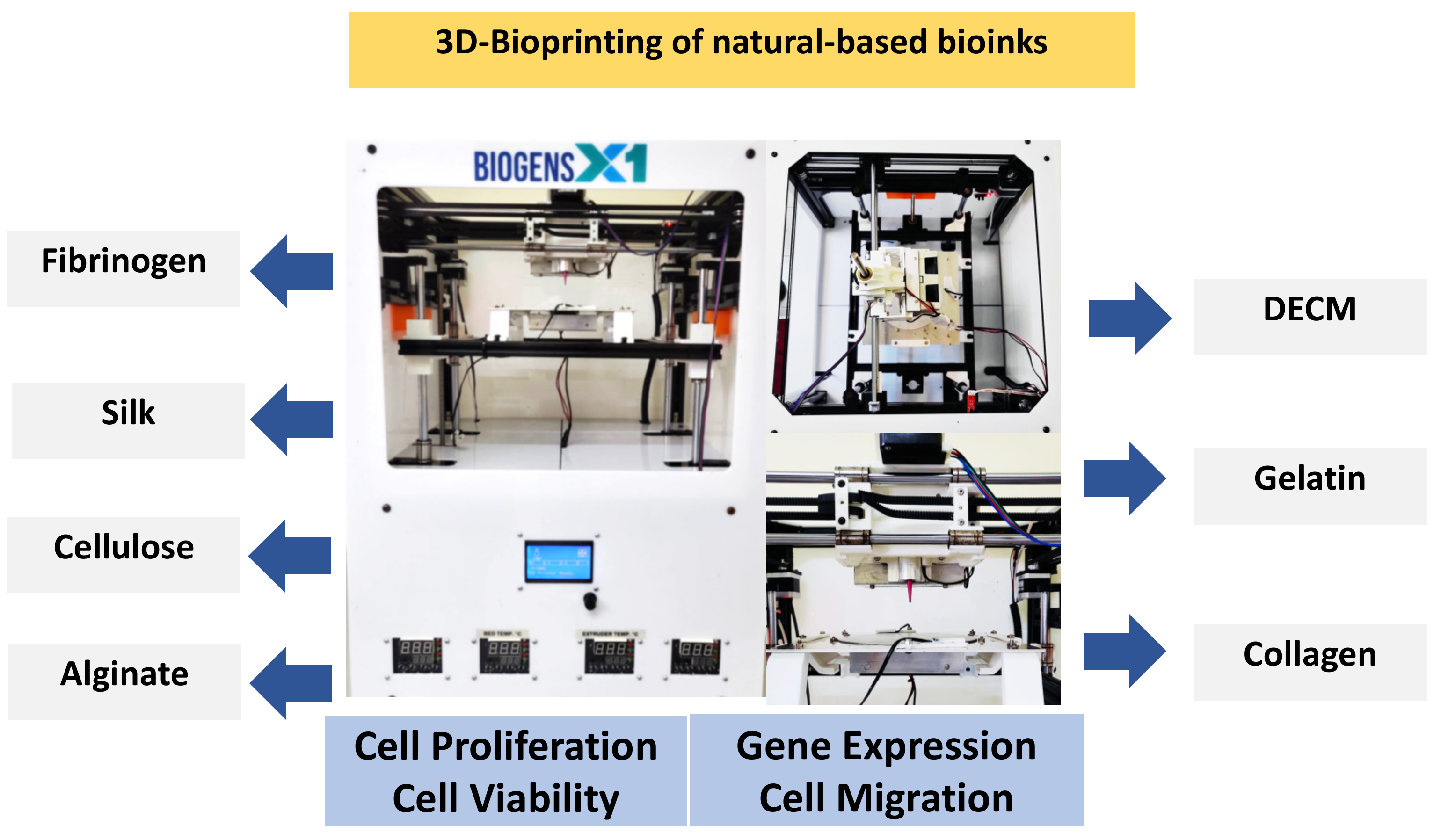
4. Natural Biomaterials
|
Type of Bioinks |
Sources |
Properties |
References |
|---|---|---|---|
|
DECM |
Majority composed of ECM |
dECM-based bioinks have viscoelastic behavior and rheological properties of dECMs, including shear viscosity and shear modulus that can preserve cells during printing. Besides, it is a biodegradable and low cytotoxicity biomaterials. |
|
|
Collagen |
Bovine, porcine, murine, and marine |
Low viscosity, high shear stress, low viscosity, and weak mechanical strength. |
|
|
Gelatin |
Bovine, porcine |
Has controllable mechanical properties depending on the concentrations, temperature-dependent, reversible state from solid to gel, and its challenging to optimize the temperature and its viscosity |
|
|
Alginate |
Algae |
has high shear-thinning properties and a faster polymerization time after printing. However, alginate do not have cell adhesion sites |
|
|
Cellulose |
Plant or bacterial ECM |
Naturally occurring, biocompatible, biodegradable, and abundant biopolymer, high solubility in water and numerous carboxyl groups |
|
|
Silk |
Silkworms and spiders |
low concentration and viscosity, slow biodegradation rate |
|
|
Fibrinogen |
Plasma protein |
Biocompatibility, biodegradability, adjustable mechanical properties, nanofibrous structural characteristics, and low viscosity properties |
|
|
Chitosan |
Chitin |
Biocompatibility, antibacterial properties, thermosensitive, and low mechanical strength |
This entry is adapted from the peer-reviewed paper 10.3390/ijms23010476
References
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burns Trauma 2018, 6, 1–10.
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situformation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780.
- Maniţă, P.G.; García-Orue, I.; Santos-Vizcaíno, E.; Hernández, R.M.; Igartua, M. 3D Bioprinting of Functional Skin Substitutes for Chronic Wound Treatment: From Current Achievements to Future Goals. SSRN Electron. J. 2020, 14, 25.
- Patel, M.; Lantis, J.C., II. Fish skin acellular dermal matrix: Potential in the treatment of chronic wounds. Chronic Wound Care Manag. Res. 2019, 6, 59–70.
- Tort, S.; Demiröz, F.T.; Coşkun Cevher, Ş.; Sarıbaş, S.; Özoğul, C.; Acartürk, F. The effect of a new wound dressing on wound healing: Biochemical and histopathological evaluation. Burns 2020, 46, 143–155.
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin. Drug Deliv. 2021, 18, 737–759.
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610.
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270.
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358.
- Jara, C.P.; Wang, O.; Paulino do Prado, T.; Ismail, A.; Fabian, F.M.; Li, H.; Velloso, L.A.; Carlson, M.A.; Burgess, W.; Lei, Y.; et al. Novel fibrin-fibronectin matrix accelerates mice skin wound healing. Bioact. Mater. 2020, 5, 949–962.
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735.
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622.
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1901502.
- Sallehuddin, N.; Nordin, A.; Idrus, R.B.H.; Fauzi, M.B. Nigella sativa and its active compound, thymoquinone, accelerate wound healing in an in vivo animal model: A comprehensive review. Int. J. Environ. Res. Public Health 2020, 17, 4160.
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33.
- Ezhilarasu, H.; Vishalli, D.; Dheen, S.T.; Bay, B.H.; Kumar Srinivasan, D. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials 2020, 10, 1234.
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477.
- Kim, M.H.; Liu, W.; Borjesson, D.L.; Curry, F.R.E.; Miller, L.S.; Cheung, A.L.; Liu, F.T.; Isseroff, R.R.; Simon, S.I. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Investig. Dermatol. 2008, 128, 1812–1820.
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979.
- Javaid, M.; Haleem, A. 3D bioprinting applications for the printing of skin: A brief study. Sens. Int. 2021, 2, 100123.
- Correia Carreira, S.; Begum, R.; Perriman, A.W. 3D Bioprinting: The Emergence of Programmable Biodesign. Adv. Healthc. Mater. 2020, 9, 1900554.
- Masri, S.; Fauzi, M. Current Insight of Printability Quality Improvement Strategies in Natural-Based Bioinks for Skin Regeneration and wound healing. Polymers 2021, 13, 1011.
- Salleh, A.; Fauzi, M.B. The in vivo, in vitro and in ovo evaluation of quantum dots in wound healing: A review. Polymers 2021, 13, 191.
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525.
- Stunova, A.; Vistejnova, L. Dermal fibroblasts—A heterogeneous population with regulatory function in wound healing. Cytokine Growth Factor Rev. 2018, 39, 137–150.
- Salimian Rizi, V. Ce Pte Us Pt. Mater. Res. Express 2019, 1–12.
- Chowdhury, S.R.; Jing, L.S.; Zolkafli, M.N.H.B.; Zarin, N.A.B.M.A.; Abdullah, W.A.B.W.; Md Mothar, N.A.B.; Maarof, M.; Abdullah, N.A.H. Exploring the potential of dermal fibroblast conditioned medium on skin wound healing and anti-ageing. Sains Malays. 2019, 48, 637–644.
- Bader, D.L.; Worsley, P.R. Technologies to monitor the health of loaded skin tissues. Biomed. Eng. Online 2018, 17, 40.
- Woo, W.M. Skin structure and biology. Augment. Cust. Strateg. CRM Digit. Age. 2019, pp. 1–14. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9783527814633.ch1 (accessed on 13 November 2021).
- Lee, H.R.; Park, J.A.; Kim, S.; Jo, Y.; Kang, D.; Jung, S. 3D microextrusion-inkjet hybrid printing of structured human skin equivalents. Bioprinting 2021, 22, e00143.
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239.
- Desanlis, A.; Albouy, M.; Rousselle, P.; Thepot, A.; Desanlis, A.; Albouy, M.; Rousselle, P.; Thepot, A.; Santos, M. Dos Validation of an implantable bioink using mechanical extraction of human skin cells: First steps to a 3D bioprinting treatment of deep second degree burn. J. Tissue Eng. Regen. Med. 2021, 15, 37–48.
- Seet, W.T.; Maarof, M.; Anuar, K.K.; Chua, K.; Wahab, A.; Irfan, A.; Ng, M.H.; Aminuddin, B.S.; Hj, B.; Ruszymah, I. Shelf-Life Evaluation of Bilayered Human Skin Equivalent, MyDermTM. PLoS ONE 2012, 7, e40978.
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction? Burn. Trauma 2019, 7, 1–12.
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92.
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53.
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprinting—Bioprinting from benchside to bedside? Acta Biomater. 2020, 101, 14–25.
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380.
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Biomaterials Science. Biomater. Sci. 2021, 9, 535–573.
- Xu, J.; Zheng, S.; Hu, X.; Li, L.; Li, W.; Parungao, R.; Wang, Y.; Nie, Y.; Liu, T.; Song, K. Advances in the Research of Bioinks Based on Natural Collagen, Polysaccharide and Their Derivatives for Skin 3D Bioprinting. Polymers 2020, 12, 1237.
- Ahadian, S.; Khademhosseini, A. Handheld Skin Printer: In-Situ Formation of Planar Biomaterials and Tissues. Physiol. Behav. 2019, 176, 139–148.
- Khoshnood, N.; Zamanian, A. Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting 2020, 20, e00095.
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797.
- Sheehy, E.J.; Cunniffe, G.M.; Brien, F.J.O. Collagen-Based Biomaterials for Tissue Regeneration and Repair 5. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018.
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147.
- Dalby, M.J. Materials Today Bio A tough act to follow: Collagen hydrogel modi fi cations to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098.
- Jang, K.S.; Park, S.J.; Choi, J.J.; Kim, H.N.; Shim, K.M.; Kim, M.J.; Jang, I.H.; Jin, S.W.; Kang, S.S.; Kim, S.E.; et al. Therapeutic efficacy of artificial skin produced by 3d bioprinting. Materials 2021, 14, 5177.
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428.
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D. Biomater. Res. 2018, 22, 11.
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015, 78, 72–78.
- Mohamed, A.L.; Soliman, A.A.F.; Abobakr, E.; Abou-zeid, N.Y.; Nada, A.A. International Journal of Biological Macromolecules Hydrogel bioink based on clickable cellulose derivatives: Synthesis, characterization and in vitro assessment. Int. J. Biol. Macromol. 2020, 163, 888–897.
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51.
- Pérez-Rigueiro, J.; Elices, M.; Plaza, G.R.; Guinea, G.V. Similarities and differences in the supramolecular organization of silkworm and spider silk. Macromolecules 2007, 40, 5360–5365.
- Mabrouk, M.; El-bassyouni, G.T.; Beherei, H.H. Inorganic additives to augment the mechanical properties of 3D-printed systems. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020.
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84.
- Daikuara, L.Y.; Chen, X.; Yue, Z.; Skropeta, D.; Wood, F.M.; Fear, M.W.; Wallace, G.G. 3D Bioprinting Constructs to Facilitate Skin Regeneration. Adv. Funct. Mater. 2021, 2105080.
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current trends on protein driven bioinks for 3d printing. Pharmaceutics 2021, 13, 1444.
- Xu, J.; Fang, H.; Zheng, S.; Li, L.; Jiao, Z.; Wang, H.; Nie, Y.; Liu, T.; Song, K. A biological functional hybrid scaffold based on decellularized extracellular matrix/gelatin/chitosan with high biocompatibility and antibacterial activity for skin tissue engineering. Int. J. Biol. Macromol. 2021, 187, 840–849.
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C 2021, 123, 111963.
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37.
