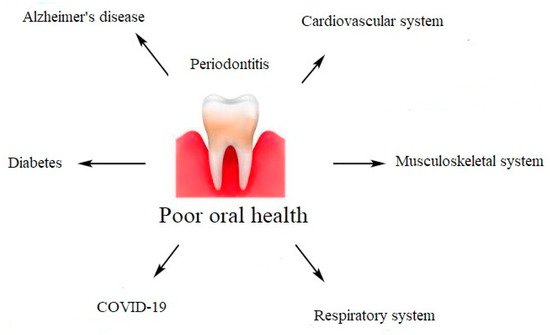
Periodontal diseases are not just simple bacterial infections, but rather complex diseases of multifactorial complexity. The connection between the subgingival microbes, the host immune, and inflammatory responses seems to be very clear. The mechanisms explaining the association between chronic periodontal disease and systemic diseases include a direct and an indirect route. The direct route results in ulceration in the lining of periodontal pockets, which can become a passage for bacteria into the systemic circulation. It leads to bacteremia, which allows bacteria to settle in distant organs aggravating existing disease conditions. The indirect route is based on the fact that chronic periodontal disease, being a significant source of inflammation, may play a role in other disease conditions in which inflammation is a major component.
1. Cardiovascular System
Cardiovascular diseases (CVD) are defined as disorders of the heart and blood vessels. An underlying cause of CVD is atherosclerosis. Atherosclerosis is a chronic, vascular inflammatory condition that results in lipids deposition in the arterial wall. The periodontal infections and cardiovascular diseases are related to each other. Many studies have shown that periodontitis usually results in higher systemic levels of C-reactive protein, interleukin (IL)-6, and neutrophils. Increasing inflammatory activity in atherosclerotic lesions, which potentially increases the risk for cardiac or cerebrovascular events may be the result of elevated inflammatory factors. Inflammation as a result of periodontitis increases systemic inflammation and oxidative stress, which contributes to and increases the already chronic inflammation present and in this way contributes to atherosclerosis and CVD [
18,
19,
20]. It has been proven that some bacteria isolated from oral cavity may be associated with platelet aggregation, as far as bacterial infections may also contribute to the acute thromboembolic events. Oral bacteria involved in periodontal disease can infect blood vessels or in some other way promote plaque formation and, thus, CVD [
21].
2. Musculoskeletal System
Chronic periodontitis is associated with a higher risk of suffering from rheumatoid arthritis. The interrelationship between systemic osteoporosis, oral bone loss, tooth loss, and risk factors for these conditions has been observed, because a remarkable similarity in the pathogenesis of periodontal diseases and rheumatoid arthritis exists. In addition, periodontal disease is thought to be an initiative factor of the autoimmune inflammatory response, which is highly connected with rheumatoid arthritis [
22,
23,
24].
3. Respiratory System and SARS-CoV-2 Infection
Poor oral hygiene and periodontitis influence the incidence of pulmonary infections. The link between dental plaque and SARS-CoV-2 infection is based on direct and indirect mechanisms. The direct mechanism is related with angiotensin-converting enzyme II (ACE-2) receptors and is associated with greater expression of ACE-2 receptors by the altered oral microbiome [
25]. It is known that ACE-2 receptors play a key role in SARS-CoV-2 entry into host cells. Therefore, a greater expression of ACE-2 receptors promotes SARS-CoV-2 infection on the epithelial cells of oral mucosa. Moreover, a colonization of dental plaque by respiratory pathogens could lead to aspiration of oral bacteria capable of causing pneumonia into the lungs. Other known mechanisms in periodontitis include alteration of the mucus surface by salivary enzymes, destruction of salivary pellicles by periodontal disease-associated enzymes and alteration of respiratory epithelium by cytokines from periodontal disease, also facilitating the infection of the epithelium by respiratory pathogens [
20,
26,
27]. The oral cavity constitutes a reservoir for respiratory pathogens, therefore patients with dental plaque or periodontitis may be more likely to develop severe pneumonia [
28,
29,
30]. The second, indirect mechanism is related to inflammatory pathways and bacterial superinfections [
29]. The greater synthesis of cytokines and chemokines appears in the gingiva, which lead to increased levels of proinflammatory cytokines in the patient’s serum. Cytokines may alter the respiratory epithelium and lead to infection by respiratory pathogens such as SARS-CoV-2. Virus activates an immune response causing a “cytokine storm” [
31]. Due to cytokine release syndrome, the hypercytokinemia is responsible for complications such as lung injury, hypercoagulation, multiorgan failure, and shock, during COVID-19. The inflammatory pathways that are involved in conditions such as diabetes, hypertension, obesity, or cardiovascular disease are the same that we observe in periodontal diseases. It is suggested, that there is a strong association between main comorbidities and increased risk of complications and death from COVID-19, and it may be connected with altered oral biofilms and periodontal disease. Moreover, indirect mechanism is based on bacterial superinfections. It is clear that altered microbiom lead to aspiration of microorganisms associated with oral diseases into lower respiratory tract and cause respiratory infections and potentially post-viral bacterial complications [
13,
32].
4. Diabetes
Studies have shown that periodontal disease may be a risk factor for diabetes, but it is suggested that the relationship between diabetes and periodontal disease runs both ways: chronic infection and inflammatory response [
21]. Severe periodontal disease can increase glucose levels, contributing to increased periods of time when the body functions with hyperglycemia. This puts people with diabetes at increased risk for diabetic complications. Pancreas cells responsible for insulin production can be damaged or destroyed by the chronic high levels of cytokines. It may induce Type 2 diabetes, even in otherwise healthy individuals with no other risk factors for diabetes. Therefore, periodontal diseases are responsible for aggravate insulin resistance and affect glycemic control [
33,
34,
35,
36,
37,
38].
5. Alzheimer’s Disease
Recent comprehensive oral-health studies demonstrate the relationship between oral pathogen, inflammation, and Alzheimer’s disease (AD). In response to oral bacterial infection, pro-inflammatory cytokines are produced by the host [
39]. Therefore, the increased level of cytokines lead to inflammation and may contribute to the brain inflammation that occurs among patients with Alzheimer’s disease. Moreover, dental plaque leads to periodontal diseases and changed microbiome. Periodontal pathogens such as
Porphyromonas gingivalis and
Treponema dentricola produce lipopolysaccharide (LPS). LPS constitutes a virulence factor and plays an important role in brain inflammatory process. Therefore, inflammation is a major factor responsible for neurodegeneration among patients [
40]. Besides LPS, also gingipains are released by
Porphyromonas gingivalis. Gingipains are classified as collagenases and trypsin-like cysteine proteinases and they are secreted by all strains of
Porphyromonas gingivalis. Gingipains together with LPS can proteolytically activate kinases such as glycogen synthase kinase-3 β (GSK-3β), which phosphorylates neuronal tau protein [
41]. Phosphorylated tau is an important agent, especially since the intraneuronal cytoskeletal alterations precede the formation of amyloid in patients with AD [
42]. Additionally, periodontal pathogens such as
Treponema dentricola and
Chlamydia pneumonia were detected in postmortem brains derived from patients with Alzheimer’s disease. Moreover,
Porphyromonas gingivalis is responsible for the peripheral and cerebral immune responses. Therefore, researchers put forward a hypothesis, that pathogens from oral cavity may invade the brain by crossing the brain–blood barrier. Periodontal pathogens may be associated with symptoms of Alzheimer’s disease [
34].
Figure 2. Impact of poor oral health on systemic diseases.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27010271