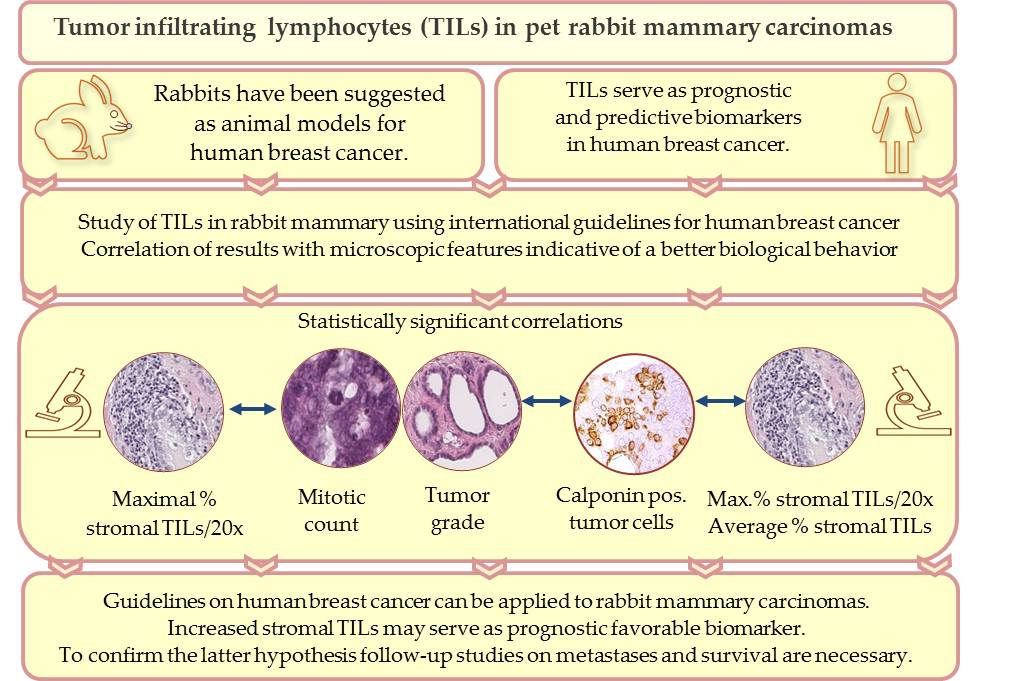
Tumor infiltrating lymphocytes (TILs) are key components of the tumor microenvironment that mediate the anti-tumor immune response. In breast cancer of humans, TILs represent prognostic and predictive biomarkers. For their standardized evaluation in routinely (hematoxylin and eosin) stained tissue sections, international guidelines exist. Recently, TILs have also been analyzed in pet rabbit mammary carcinomas according to these international guidelines. Results of the study on rabbit mammary carcinomas showed a statistically significant association between higher TIL numbers in stromal TIL hotspot areas and microscopic parameters indicative of a better tumor differentiation, i.e. decreased mitotic count, lower histological tumor grade and higher percentage of calponin positive tumor cells. These findings suggest that in rabbit mammary carcinomas TIL hotspot areas may exert an influence on the biological behavior of these tumors. The present study contributes to comparative pathology. In addition, it provides the basis for further investigations into the impact of TILs on clinical parameters of pet rabbit mammary carcinomas.
- comparative pathology
- histopathology
- mammary carcinoma
- pet rabbits
- translational medicine
- tumor infiltrating lymphocytes
1. Graphical abstract
2. Introduction
Nowadays, rabbits are popular pets[1] and mammary carcinomas are quite commonly diagnosed in does[2][3]. In women, breast carcinoma is worldwide the most frequent cancer type[4][5][6].
Studies revealed comparative features of mammary tumors of pet rabbits and breast cancer in humans, and pet rabbits have been proposed as suitable animal models for certain types of breast cancer[3][7][8].
Majority of breast cancer cases in humans[4] and mammary carcinomas in pet rabbits[7][8][9][10] are histologically diagnosed as invasive adenocarcinomas. The histological grading scheme of Elston and Ellis[11] is used to determine the differentiation of mammary carcinomas in humans[12] as well as in rabbits[7][9]. Immunostaining for estrogen receptor-α (ER-α) and progesterone receptor (PR) reveals expression of these hormone receptors in molecular subtypes of human[4][12] and in rabbit mammary carcinomas[7]. The disease affects most frequently middle aged to older individuals: In women, 80% of breast cancer cases are diagnosed at an age ≥50 years[5][13],and the average age of does with mammary carcinomas is 4.9-5.5 years[7][9][10]. The reproductive age of female rabbits starts with 4-6 months, and the life expectancy of pet rabbits is 6-13 years[14]. In rabbits, elevated serum prolactin concentrations are associated with benign proliferative mammary gland lesions [15,16,17] that may undergo neoplastic transformation in rare cases [17]. In women a rise in prolactin for a longer duration represents a predisposing factor for breast cancer development [18]. Investigations of mammary tumor development in breeding colonies of rabbits showed clustering in distinct families suggesting a genetic predisposition [19]. In humans, familial breast cancer accounts for approx. 7% of all breast cancer cases [20], and it is in 25% attributed to mutations in breast cancer gene 1 and 2 (BRCA 1; BRCA 2) [6].
Close similarities between the immune system of rabbits and humans [21,22] facilitate the use of the rabbit in translational immunological studies [21,22,23,24] that include immuno-oncological research [25,26].
The fate of a tumor (regression, progression, metastases) is markedly influenced by the hosts immune response that include components of the innate and adaptive immunity [25,27].
In breast cancer of humans, it has been shown that the general evaluation of tumor infiltrating lymphocytes (TILs) without subclassification has prognostic significance [28,29,30] and can aid in treatment decisions [28,29,31]. International guidelines facilitate a comparison between different studies [28,29].
References
- DeMello M. Rabbits multiplying like rabbits. The rise in the worldwide popularity of rabbits as pets. In Companion animals in everyday life. Situating animal-human engagement within cultures; Pregowski PM, Eds.; Palgrave MacMillan, Springer Nature: New York, USA, 2016; pp. 91-108.
- Barthold SW, Griffey SM, Percy DH . Pathology of laboratory rodents and rabbits. 4th edition; Barthold SW, Griffey SM, Percy DH , Eds.; John Wiley & Sons: Chinchester, UK, 2016; pp. 253-323.
- Schöniger S, Degner S, Jasani B, Schoon H-A; A Review on Mammary Tumors in Rabbits: Translation of Pathology into Medical Care. Animals 2019, 9, 762, 10.3390/ani9100762.
- Makki, J; Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clinical Medicine Insights: Pathology 2015, 8, 23-31, 10.4137/cpath.s31563.
- Kamińska M, Ciszewski T, Łopacka-Szatan K, Miotła P, Starosławska E; Breast cancer risk factors. Menopausal Review 2015, 3, 196-202, 10.5114/pm.2015.54346.
- Hawsawi JM, Al-Numair NS, Sobahy TM, Al-Ajmi AM, Al-Harbi RM, Baghdadi MA, Oyouni A, Alamer OM; The role of BRCA1/2 in hereditary and familial breast and ovarian cancers.. Molecular Genetics & Genomic Medicine 2019, 7, e879, 10.1002/mgg3.879.
- Degner S, Schoon H-A, Laik-Schandelmaier C, Aupperle-Lellbach H, Schöniger S; Estrogen Receptor–α and Progesterone Receptor Expression in Mammary Proliferative Lesions of Female Pet Rabbits. Veterinary Pathology 2018, 55, 838-848, 10.1177/0300985818788611.
- Degner S, Schoon H-A, Degner S, Baudis M, Schandelmaier C, Aupperle-Lellbach H, Schöniger S; Expression of myoepithelial markers in mammary carcinomas of 119 pet rabbits. Animals 2019, 9, 740, 10.3390/ani9100740.
- Schöniger S, Schoon H-A, Horn L-C; Tumors and Tumor-like Lesions in the Mammary Gland of 24 Pet Rabbits. Veterinary Pathology 2013, 51, 569-580, 10.1177/0300985813497486.
- Baum B, Hewicker-Trautwein M; Classification and Epidemiology of Mammary Tumours in Pet Rabbits (Oryctolagus cuniculus). Journal of Comparative Pathology 2015, 152, 291-298, 10.1016/j.jcpa.2015.01.009.
- Elston CW, Ellis I; pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19, 403-410, 10.1111/j.1365-2559.1991.tb00229.x.
- Ellis IO, Lee AHE, Pinder SE, Rakha EA . Tumors of the breast. In Diagnostic histopathology of tumors, 4th edition; Fletcher, CDM, Eds.; Elsevier, Saunders: Philadelphia, PA, USA, 2013; pp. 1057-1145.
- Benz CC; Impact of aging on the biology of breast cancer. Critical Reviews in Oncology/Hematology 2008, 66, 65-74, 10.1016/j.critrevonc.2007.09.001.
- Müller K, Schall H . Kaninchen. In Krankheiten der Heimtiere. 8th edition; Fehr M, Sassenburg L, Zwart P , Eds.; Schlütersche Verlag: Hannover, Germany, 2014; pp. 1-56.