The effectiveness of opioids in the treatment of neuropathic pain is limited. It was demonstrated that magnesium ions (Mg2+), physiological antagonists of N-methyl-D-aspartate receptor (NMDAR), increase opioid analgesia in chronic pain. Our study aimed to determine the molecular mechanism of this action. Early data indicate the cross-regulation of mu-opioid receptor (MOR) and NMDAR in pain control. Morphine acting on MOR stimulates protein kinase C (PKC), while induction of NMDAR (for example in a state of neuropathic pain) recruits protein kinase A (PKA) leading to disruption of the MOR-NMDAR complex and promoting functional changes in receptors. The level of phosphorylated NMDAR NR1 subunit (pNR1) and phosphorylated MOR (pMOR) in the periaqueductal gray matter was determined with the Western blot method. The activity of PKA and PKC was examined by standard enzyme immunoassays. Mg2+ administered alone significantly decreased the level of pNR1 and pMOR, and activity of both tested kinases. The results suggest that blocking NMDAR signaling by Mg2+ restores the MOR-NMDAR complex and thus enables morphine analgesia in neuropathic rats.
- magnesium
- morphine
- N-methyl-D-aspartate receptor
- µ-opioid receptor
- receptor association
- analgesia
- neuropathic rats
1. Introduction
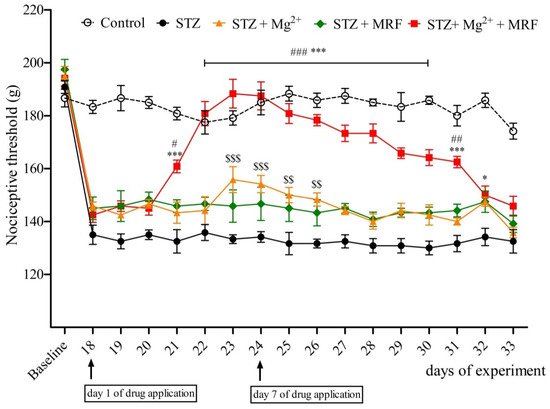
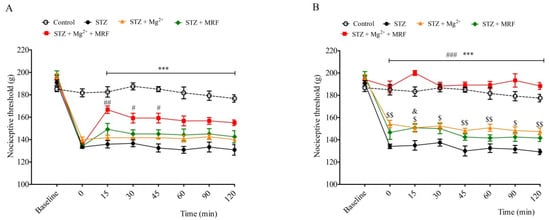
2. The Influence of Mg2+ on the Analgesic Effect of Morphine in Streptozotocin-Induced Hyperalgesia after Mechanical Stimulation
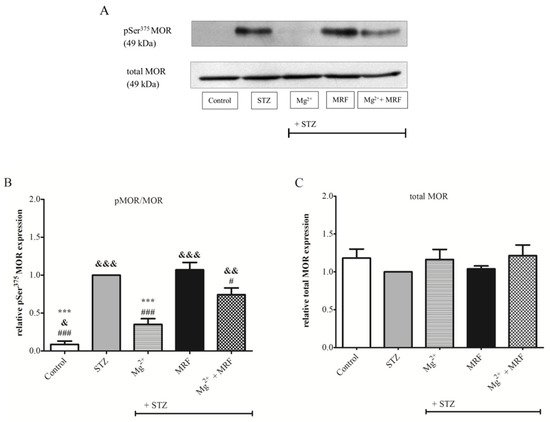
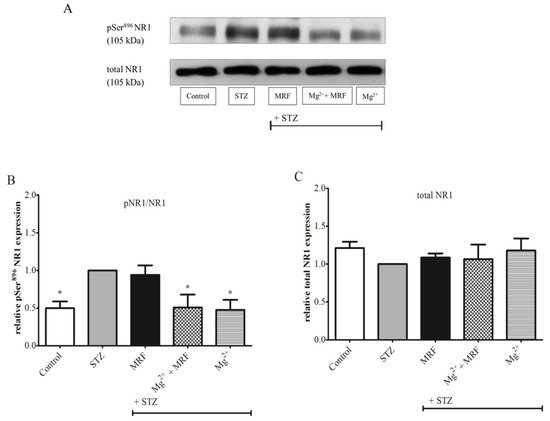
3. Changes in the Expression of Phosphorylated Proteins Measured at the Serine Residues of N-methyl-D-aspartate Receptor NR1 Subunit and µ Opioid Receptor
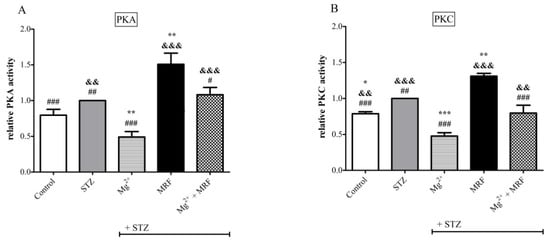
4. Changes in the Activity of Protein Kinase A and Protein Kinase C in the Streptozotocin-Treated Rats after Administration of Tested Compounds
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413599
References
- S. Arnér; B. A. Meyerson; Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain 1988, 33, 11-23, 10.1016/0304-3959(88)90198-4.
- Luigi Morrone; Damiana Scuteri; Laura Rombola; Hirokazu Mizoguchi; Giacinto Bagetta; Opioids Resistance in Chronic Pain Management. Current Neuropharmacology 2017, 15, 444-456, 10.2174/1570159x14666161101092822.
- Ajay S. Yekkirala; David P. Roberson; Bruce P. Bean; Clifford J. Woolf; Breaking barriers to novel analgesic drug development. Nature Reviews Drug Discovery 2017, 16, 545-564, 10.1038/nrd.2017.87.
- A. H. Dickenson; NMDA receptor antagonists: interactions with opioids. Acta Anaesthesiologica Scandinavica 1997, 41, 112-115, 10.1111/j.1399-6576.1997.tb04624.x.
- Jianren Mao; NMDA and opioid receptors: their interactions in antinociception, tolerance and neuroplasticity. Brain Research Reviews 1999, 30, 289-304, 10.1016/s0165-0173(99)00020-x.
- Russell K. Portenoy; Kathleen M. Foley; Charles E. Inturrisi; The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain 1990, 43, 273-286, 10.1016/0304-3959(90)90025-9.
- Patricia Lavand'Homme; Arnaud Steyaert; Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Practice & Research Clinical Anaesthesiology 2017, 31, 487-498, 10.1016/j.bpa.2017.05.003.
- Sebastiano Mercadante; Edoardo Arcuri; Angela Santoni; Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019, 33, 943-955, 10.1007/s40263-019-00660-0.
- J. Nee; V. Rangan; A. Lembo; Reduction in pain: Is it worth the gain? The effect of opioids on the GI tract. Neurogastroenterology & Motility 2018, 30, e13367, 10.1111/nmo.13367.
- Eugene A. Kiyatkin; Respiratory depression and brain hypoxia induced by opioid drugs: Morphine, oxycodone, heroin, and fentanyl. Neuropharmacology 2019, 151, 219-226, 10.1016/j.neuropharm.2019.02.008.
- Sophie Begon; Gisèle Pickering; Alain Eschalier; Claude Dubray; Magnesium and MK-801 have a similar effect in two experimental models of neuropathic pain. Brain Research 2000, 887, 436-439, 10.1016/s0006-8993(00)03028-6.
- Jianren Mao; Donald Price; Ronald L. Hayes; Juan Lu; David J. Mayer; Hanan Frenk; Intrathecal treatment with dextrorphan or ketamine potently reduces pain-related behaviors in a rat model of peripheral mononeuropathy. Brain Research 1993, 605, 164-168, 10.1016/0006-8993(93)91368-3.
- Hirokazu Mizoguchi; Chizuko Watanabe; Akihiko Yonezawa; Shinobu Sakurada; Chapter 19 New Therapy for Neuropathic Pain. International Review of Neurobiology 2009, 85, 249-260, 10.1016/s0074-7742(09)85019-8.
- Christine N Sang; NMDA-Receptor Antagonists in Neuropathic Pain: Experimental Methods to Clinical Trials. Journal of Pain and Symptom Management 2000, 19, 21-25, 10.1016/s0885-3924(99)00125-6.
- Maarten Swartjes; Aurora Morariu; Marieke Niesters; Leon Aarts; Albert Dahan; Nonselective and NR2B-selective N -methyl-d-aspartic Acid Receptor Antagonists Produce Antinociception and Long-term Relief of Allodynia in Acute and Neuropathic Pain. Anesthesiology 2011, 115, 165-174, 10.1097/aln.0b013e31821bdb9b.
- Valéria Martinez; Dennis Christensen; Valérie Kayser; The glycine/NMDA receptor antagonist (+)-HA966 enhances the peripheral effect of morphine in neuropathic rats. Pain 2002, 99, 537-545, 10.1016/s0304-3959(02)00270-1.
- Michael L Nichols; Yvan Lopez; Michael H Ossipov; Di Bian; Frank Porreca; Enhancement of the antiallodynic and antinociceptive efficacy of spinal morphine by antisera to dynorphin A (1–13) or MK-801 in a nerve-ligation model of peripheral neuropathy. Pain 1997, 69, 317-322, 10.1016/s0304-3959(96)03282-4.
- Tatsuo Yamamoto; Tony L. Yaksh; Studies on the spinal interaction of morphine and the NMDA antagonist MK-801 on the hyperesthesia observed in a rat model of sciatic mononeuropathy. Neuroscience Letters 1992, 135, 67-70, 10.1016/0304-3940(92)90137-v.
- L. J. Rondón; A. M. Privat; L. Daulhac; N. Davin; A. Mazur; J. Fialip; A. Eschalier; C. Courteix; Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. The Journal of Physiology 2010, 588, 4205-4215, 10.1113/jphysiol.2010.197004.
- Wen-Hua Xiao; Gary J. Bennett; Magnesium suppresses neuropathic pain responses in rats via a spinal site of action. Brain Research 1994, 666, 168-172, 10.1016/0006-8993(94)90768-4.
- S Brill; P M Sedgwick; W Hamann; P P Di Vadi; Efficacy of intravenous magnesium in neuropathic pain.. British Journal of Anaesthesia 2002, 89, 711-4, .
- A. A. Yousef; A. E. Al‐Deeb; A double‐blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia 2012, 68, 260-266, 10.1111/anae.12107.
- Sophie Begon; Gisèle Pickering; Alain Eschalier; Claude DuBray; Magnesium Increases Morphine Analgesic Effect in Different Experimental Models of Pain. Anesthesiology 2002, 96, 627-632, 10.1097/00000542-200203000-00019.
- Magdalena Bujalska.; Helena Makulska-Nowak.; Stanisław W. Gumułka; Magnesium ions and opioid agonists in vincristine-induced neuropathy. Pharmacological Reports 2009, 61, 1096-1104, 10.1016/s1734-1140(09)70172-0.
- Magdalena Bujalska; Ewelina Malinowska; Helena Makulska-Nowak; Stanisław Witold Gumułka; Magnesium Ions and Opioid Agonist Activity in Streptozotocin-Induced Hyperalgesia. Pharmacology 2008, 82, 180-186, 10.1159/000151346.
- Ahmet Ulugol; Aysegul Aslantas; Yesim Ipci; Alev Tuncer; Cetin Hakan Karadag; Ismet Dokmeci; Combined systemic administration of morphine and magnesium sulfate attenuates pain-related behavior in mononeuropathic rats. Brain Research 2002, 943, 101-104, 10.1016/s0006-8993(02)02618-5.
- John T. Williams; Susan L. Ingram; Graeme Henderson; Charles Chavkin; Mark Von Zastrow; Stefan Schulz; Thomas Koch; Christopher J. Evans; Macdonald J. Christie; Regulation of µ-Opioid Receptors: Desensitization, Phosphorylation, Internalization, and Tolerance. Pharmacological Reviews 2013, 65, 223-254, 10.1124/pr.112.005942.
- Anika Mann; Susann Illing; Elke Miess; Stefan Schulz; Different mechanisms of homologous and heterologous μ-opioid receptor phosphorylation. British Journal of Pharmacology 2014, 172, 311-316, 10.1111/bph.12627.
- Stefan Schulz; Dana Mayer; Manuela Pfeiffer; Ralf Stumm; Thomas Koch; Volker Höllt; Morphine induces terminal μ-opioid receptor desensitization by sustained phosphorylation of serine-375. The EMBO Journal 2004, 23, 3282-3289, 10.1038/sj.emboj.7600334.
- Kasper B. Hansen; Feng Yi; Riley Perszyk; Hiro Furukawa; Lonnie P. Wollmuth; Alasdair Gibb; Stephen F. Traynelis; Structure, function, and allosteric modulation of NMDA receptors. Journal of General Physiology 2018, 150, 1081-1105, 10.1085/jgp.201812032.
- Pierre Paoletti; Jacques Neyton; NMDA receptor subunits: function and pharmacology. Current Opinion in Pharmacology 2007, 7, 39-47, 10.1016/j.coph.2006.08.011.
- Whittemore G. Tingley; Michael D. Ehlers; Kimihiko Kameyama; Carol Doherty; Janine B. Ptak; Clark T. Riley; Richard L. Huganir; Characterization of Protein Kinase A and Protein Kinase C Phosphorylation of the N-Methyl-D-aspartate Receptor NR1 Subunit Using Phosphorylation Site-specific Antibodies. Journal of Biological Chemistry 1997, 272, 5157-5166, 10.1074/jbc.272.8.5157.
- John Q. Wang; Ming-Lei Guo; Dao-Zhong Jin; Bing Xue; Eugene E. Fibuch; Li-Min Mao; Roles of subunit phosphorylation in regulating glutamate receptor function. European Journal of Pharmacology 2013, 728, 183-187, 10.1016/j.ejphar.2013.11.019.
- Jian-Yu Lan; Vytenis A. Skeberdis; Teresa Jover; Sonja Y. Grooms; Ying Lin; Ricardo Araneda; Xin Zheng; Michael V. L. Bennett; R. Suzanne Zukin; Protein kinase C modulates NMDA receptor trafficking and gating. Nature Neuroscience 2001, 4, 382-390, 10.1038/86028.
- Xiaoju Zou; Qing Lin; William D. Willis; Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Research 2004, 1020, 95-105, 10.1016/j.brainres.2004.06.017.
- L.-M Kow; K.G Commons; S Ogawa; D.W Pfaff; Potentiation of the excitatory action of NMDA in ventrolateral periaqueductal gray by the μ-opioid receptor agonist, DAMGO. Brain Research 2002, 935, 87-102, 10.1016/s0006-8993(02)02532-5.
- Susumu Koyama; Norio Akaike; Activation of μ-opioid receptor selectively potentiates NMDA-induced outward currents in rat locus coeruleus neurons. Neuroscience Research 2008, 60, 22-28, 10.1016/j.neures.2007.09.003.
- Gilles Martin; Zhiguo Nie; George Robert Siggins; μ-Opioid Receptors Modulate NMDA Receptor-Mediated Responses in Nucleus Accumbens Neurons. The Journal of Neuroscience 1997, 17, 11-22, 10.1523/JNEUROSCI.17-01-00011.1997.
- María Rodríguez-Muñoz; Pilar Sánchez-Blázquez; Ana Vicente-Sánchez; Esther Berrocoso; Javier Garzón; The Mu-Opioid Receptor and the NMDA Receptor Associate in PAG Neurons: Implications in Pain Control. Neuropsychopharmacology 2011, 37, 338-349, 10.1038/npp.2011.155.
- Pilar Sánchez-Blázquez; Maria Rodríguez-Muñoz; Esther Berrocoso; Javier Garzón; The plasticity of the association between mu-opioid receptor and glutamate ionotropic receptor N in opioid analgesic tolerance and neuropathic pain. European Journal of Pharmacology 2013, 716, 94-105, 10.1016/j.ejphar.2013.01.066.
- Pilar Sánchez-Blázquez; María Rodríguez-Muñoz; Javier Garzón; Mu-Opioid Receptors Transiently Activate the Akt-nNOS Pathway to Produce Sustained Potentiation of PKC-Mediated NMDAR-CaMKII Signaling. PLOS ONE 2010, 5, e11278, 10.1371/journal.pone.0011278.
- María Rodríguez-Muñoz; Elena de la Torre-Madrid; Pilar Sánchez-Blázquez; Javier Garzón; NO-released Zinc Supports the Simultaneous Binding of Raf-1 and PKCγ Cysteine-Rich Domains to HINT1 Protein at the Mu-Opioid Receptor. Antioxidants & Redox Signaling 2011, 14, 2413-2425, 10.1089/ars.2010.3511.
- María Rodríguez-Muñoz; Elena De La Torre-Madrid; Pilar Sánchez-Blázquez; Jia Bei Wang; Javier Garzón; NMDAR-nNOS generated zinc recruits PKCγ to the HINT1–RGS17 complex bound to the C terminus of Mu-opioid receptors. Cellular Signalling 2008, 20, 1855-1864, 10.1016/j.cellsig.2008.06.015.
- Pilar Sánchez-Blázquez; María Rodríguez-Muñoz; Carlos Montero; Elena De La Torre-Madrid; Javier Garzón; Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacology 2008, 54, 319-330, 10.1016/j.neuropharm.2007.10.002.
- Sascha Just; Susann Illing; Michelle Trester-Zedlitz; Elaine K. Lau; Sarah J. Kotowski; Elke Miess; Anika Mann; Christian Doll; Jonathan C. Trinidad; Alma L. Burlingame; et al. Differentiation of Opioid Drug Effects by Hierarchical Multi-Site Phosphorylation. Molecular Pharmacology 2012, 83, 633-639, 10.1124/mol.112.082875.
- Javier Garzón; María Rodríguez-Muñoz; Pilar Sánchez-Blázquez; Do pharmacological approaches that prevent opioid tolerance target different elements in the same regulatory machinery?. Current Drug Abuse Reviewse 2008, 1, 222-238, 10.2174/1874473710801020222.
