Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
Many studies suggest that vitamin D improves immune function, reducing susceptibility to infection. In contrast, an extensive number of scientific studies highlight its immunosuppressive effects. Thus, it seems that vitamin D supports immune response under physiological conditions, but it also has an active role in autoimmunity prevention. In short, its effects would depend on the immunological situation of the patient.
- vitamin D
- COVID-19
- Mechanisms
- autoimmunity
1. Vitamin D Mechanisms of Action
Its active form (1,25-dihydroxyvitamin D or calcitriol) is mainly known for regulating calcium homeostasis and bone health. However, it has also shown to play a role in the regulation of the immune system, so much that its deficient consumption is related to malfunction and dysregulation of immunity and inflammatory status [1].
A summary of vitamin D main mechanisms of action is shown in Figure 1.
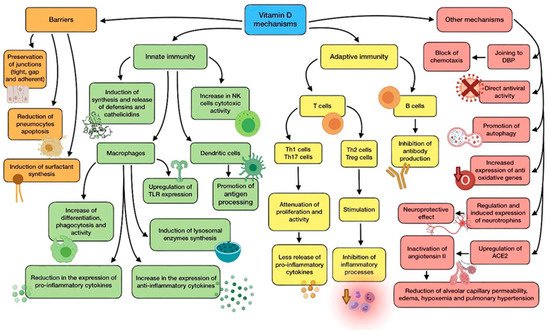
Figure 1. Summary of vitamin D main mechanisms of action.
The physiological link between vitamin D and the functioning of immune cells, such as lymphocytes, dendritic cells, and monocytes/macrophages is highly dependent, as they are able to carry out and enhance their functions after binding to calcitriol. In order to do so, these cells express CYP27B1 (1α-hydroxylase), which is responsible for vitamin D activation through the transformation of calcidiol (pre-vitamin D), into calcitriol [2].
Calcitriol interacts with the vitamin D receptor (VDR), a specific nuclear receptor which is mostly renowned for its role in the regulation of phosphorus and calcium levels, although it plays a part in both innate and adaptive immune systems. It is expressed by the previously cited immune cells, whose correct functioning, proliferation, and differentiation, therefore, depends on the bioavailability of the vitamin in them, due to this autocrine and paracrine mechanism. Airway epithelial cells also express CYP27B1, thus cooperating in the activation of immune cells. After attaching to vitamin D, activated VDR dimerise and the complex translocate to the nucleus so as to interact with regulatory sequences (VDRE) of target genes, modifying their transcription [3].
Vitamin D shows a great variety of mechanisms by which it protects against microbial infection, possibly reducing the risk of poor prognosis and death. They can be classified into three general categories: maintenance of physical barriers, improvement of innate immunity, and enhancement of adaptive immunity [4].
(a) Barriers: junction integrity may be disturbed by viruses, making invasion easier for other pathogens. In this field, vitamin D helps to preserve tight, gap, and adherent junctions between epithelial cells, reinforcing natural barriers against microorganisms [5].
Impairment in type II pneumocytes leads to a reduction in surfactant production, which increases the risk of ARDS. Vitamin D has proved to reduce pneumocyte apoptosis and induce surfactant synthesis, which would prevent serious lung injuries [6].
(b) Innate immune system: vitamin D is able to induce synthesis and release of antimicrobial peptides, such as cathelicidins (LL-37), and defensins in monocytes/macrophages [3]. Cathelicidins have demonstrated direct antimicrobial properties against a wide range of microbes, such as Gram-positive and Gram-negative bacteria, enveloped and non-enveloped viruses, and fungi. They reach these effects by altering cell membranes of infecting pathogens, as observed in a mouse model in which influenza A virus replication was reduced by cathelicidin LL-37 [7]. This protein has several other functions against microorganisms, including chemotaxis stimulation in neutrophils, monocytes and T cells, production of different cytokines, and induction of autophagy in infected epithelial cells (which boost the clearance of respiratory pathogens) [8]. As for defensins, β-defensin-2 also stimulates the expression of cytokines and chemokines related to the recruitment of immune cells. In the same way as cathelicidins, defensins are also able to block viral entry into cells [9].
In monocytes, vitamin D promotes differentiation to macrophages, increases phagocytosis, and increases superoxide output. Moreover, it can reduce the expression of pro-inflammatory cytokines and upregulate the expression of the anti-inflammatory ones, thus attenuating cytokine storm. Specially, vitamin D is able to reduce IL-6 expression through the p38 MAP kinase signaling pathway. Vitamin D also induces the synthesis of lysosomal enzymes and the release of NO, which cooperate in countering infection [10]. In dendritic cells, it promotes antigen processing, though it also might impair cell maturation and antigen presentation. In addition, vitamin D also upregulates the expression of TLR2 and TLR4, both in monocytes and dendritic cells. The interaction of molecules with the components of pathogens enhances the expression of CYP27B1 and VDR, in order to improve vitamin D-derived actions [3][11]. Besides, NK cells activity may be diminished by low-serum vitamin D, while high serum levels could significantly increase its cytotoxicity [11].
(c) Adaptive immune system: with regard to adaptive immunity, vitamin D modifies a response toward a more anti-inflammatory pattern, developing a different effect depending on the type of T cell. On the one hand, it has shown to attenuate Th1 cell proliferation and activity, which leads to a decrease in the release of pro-inflammatory cytokines that contribute to ARDS, such as TNFα, INF-γ, IL-6, and IL-2. On the other hand, vitamin D seems to stimulate Th2 cells and Treg cells, thereby inhibiting inflammatory processes. It also reduces Th17 activity and IL-17 production. Its impact on CD8+ T cells appears to be minor [12][13].
During the elicitation phase at the beginning of inflammation, vitamin D inhibits Th1, Th17, and the abnormal release of their cytokines. However, during the resolution phase, it induces Th2, Treg, and the release of theirs (specially IL-10), thus avoiding organ damage due to an excessive cytokine secreting response. In short, vitamin D promotes a balanced T cell’s defensive response against microbes [11]. As for B lymphocytes, vitamin D has also been reported to inhibit antibody production [13].
(d) Other relevant mechanisms: it has been proven through animal models that vitamin D attenuates ARDS-related to microbes by modulating the activity of the renin–angiotensin system (RAS) and the expression of angiotensin converter enzyme 1 and 2 (ACE1, ACE2). The increase in alveolar capillary permeability is one of the key mediators in this pathology, leading to pulmonary edema, pulmonary hypertension, and hypoxemia [5]. As ACE2 inactivates angiotensin II, it acts as a negative regulator of the RAS. Therefore, it may have a beneficial role in regulating vascular permeability and its pulmonary consequences during ARDS development. Calcitriol has demonstrated its ability to upregulate pulmonary ACE2 and downregulate angiotensin II and renin, so it can play a key role in hindering the progression of respiratory distress induced by infection [12]. This mechanism seems to clash somehow with SARS-CoV-2 characteristics, as it needs ACE2 to enter epithelial cells. Nevertheless, the binding of the S1 spike protein to ACE2 causes both the enzyme and the virus to be translocated into the cell, thus decreasing its surface expression and probably contributing to the development of pulmonary pathology. There seems to be an association CVD between high levels of ACE2 and survival likelihood, which in the end would be beneficial in ARDS and COVID-19 [5][14]. Furthermore, vitamin D deficiency induces activation of RAS, which may lead to CVD and reduced lung function, as well as comorbidities associated with a higher risk of severe cases of COVID-19 [14].
Vitamin D binding protein (DBP) is the main transport protein of this compound (though it can also bind actin and other molecules), and it might have an underrated role in the onset of ARDS on account of SARS-CoV-2 infection [12]. During ARDS, actin is released into extracellular fluids, and has a pro-coagulant action when it polymerizes from globular actin (G-actin) to filamentous actin (F-actin). As a response, DBP slices the F-actin actin and avoids G-actin repolymerization. However, DBP attached to G-actin acts as an indirect but relevant factor in inflammation by increasing the action of neutrophil chemoattractants. According to this, vitamin D would block chemotaxis by competing for the same binding site on DBP, so low levels of the vitamin could be correlated with a more serious outcome during infection [12].
The powerful neuroprotective effect of vitamin D should also be taken into consideration, as it could come in handy to prevent neurological symptoms produced by COVID-19. This effect is linked to regulation of neurotrophins, due to the fact that vitamin D stimulates expression of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3 (NT3), just like neurotrophin receptor p75NTR in neurons, glial cells, and Schwann cells. Ultimately, this is translated into protection against brain ischemia and neurodegenerative disorders. In addition, vitamin D has been reported to enhance remyelination of neurons by promoting the migration and differentiation of oligodendrocytes’ progenitors [15][16].
The vitamin has shown antiviral functions against a number of different microbes, including influenza virus or Epstein-Barr virus (EBV). The mechanisms involved in these functions are the production of antimicrobial peptides, blockage of viral entry, replication and induction of autophagy, induction of virus specific CD8+ T cells, and suppression of TLRs (different from TLR2 and TLR4) [11].
As for oxidative stress, vitamin D has been reported to upregulate the expression of some antioxidant genes (for example, glutathione reductase), thus reducing the amount of ROS generated because of inflammation which could contribute to ARDS and lung damage [5]. Excess of ROS production enhances NF-κB expression in immune cells, causing a raised production of pro-inflammatory cytokines and other mediators. According to this, ROS reduction promoted by vitamin D leads to a decrease in NF-κB pathway and, consequently, the genes regulated by it [3][12].
Promoting autophagy is another relevant mechanism of vitamin D against infection, as it is an essential way for cells to deal with viruses, thus enabling their degradation and subsequent antigen presentation. The specific mechanism involves downregulating the mTOR pathway (which inhibits autophagy), and upregulating Beclin-1 (BECN1) and class III phosphatidylinositol 3-kinase (PI3KC3), i.e., the key drivers of the process. As stated before, cathelicidin expression enhanced by vitamin D also induces this process [9].
Another noteworthy fact is that correction of vitamin D insufficiency significantly reduces the expression of dipeptidyl peptidase 4 (DPP-4 or CD26). This is one of the adhesion proteins through which SARS-CoV-2 is believed to get access to host cells, owing to an interaction with the S1 domain of spike glycoprotein. This suggests that it may be a prominent virulence factor in the disease [14].
2. Evidence about Vitamin D Regarding Respiratory Infections and COVID-19
Vitamin D deficiency is widely common, affecting over a billion people worldwide. Among general population, there are two groups at particular risk: individuals with darker skin and the elderly. As for the first group, increased pigmentation complicates the penetration of UV light needed for epidermal synthesis (which represents the main source of vitamin D) [11]. This might be one of the factors involved in the disproportionate increase in COVID-19 affectation among black people compared to the rest of population [17]. Besides, a similar influence was observed in studies related to the 1918–1919 influenza pandemic [18]. With regard to elder people, a combination of factors (such as reduced epidermal synthesis, increased time indoors, reduced food intake and impairment in vitamin D metabolism owing to drugs) elevates their risk of suffering from vitamin D deficiency. Needless to say, the elderly has also been the group of population most severely affected by SARS-CoV-2 infection. Researchers have highlighted the relevance for vulnerable groups to maintain adequate vitamin D levels in order to reduce the risk of respiratory tract infections, including COVID-19 [2][19]. However, this pandemic has also increased the risk for general population to develop a vitamin D deficiency, due to lockdown or “staying at home” mitigation strategies, which are adopted to prevent the virus propagation of the virus. This reduced sun exposure time makes it undoubtedly more difficult to reach an adequate vitamin D status, especially during autumn and winter.
Another noteworthy fact is that countries have experimented on a more complicated situation with regard to the pandemic during and right after seasons with less sun exposure (autumn and winter), rather than after seasons with more sun hours (spring and summer). This might be connected to the lower vitamin D concentrations that population has in those periods of time. It has been suggested that latitude may play a role as well [20]. Though the recommended dietary allowance (RDA) of vitamin D is 15–20 µg/d, scientific evidence recommends doses of 250 µg/d for a month in order to quickly raise concentrations, followed by a maintenance dose of 125 µg/d for 12 months. Higher doses would be required for vulnerable groups and cases of present infection. According to their sun exposure, supplementation in countries far from the equator could be proposed as a routine without having to check plasma levels [21].
A meta-analysis of several randomized controlled trials (RCTs) showed an overall protective effect against ARDS due to vitamin D supplementation, with the most important benefits appearing in patients with a greater deficiency of this nutrient [22]. Some meta-analyses found a strong association between low vitamin D status and risk of respiratory tract infection, even though others have not achieved the same conclusion. Consequently, the usefulness of vitamin D supplementation in viral respiratory diseases remains unclear [23].
This vitamin has been discussed specifically for its role in influenza therapy and prevention, with mixed effects attained. A Chinese RCT in which vitamin D was administered to infants reported protective effects with regard to incidence and severity of the infection. Similar results were obtained by another RCT in Japan [24][25]. In addition, systematic reviews and meta-analyses on the role of this nutrient in influenza vaccination showed low seroprotection rates in those who had vitamin D deficiency. On the other hand, some studies fail to find a correlation between supplementation and reduction in infections, although they do not demonstrate a complete inefficacy for this intervention [26].
These findings related to respiratory tract infections have led to the speculation that vitamin D may have a protective role in COVID-19 [27]. There is some discrepancy regarding vitamin D deficiency as an independent risk factor for the disease, as poor prognosis would possibly be due to a combination of factors, among which vitamin D hypovitaminosis would be included. This situation is likely in individuals with obesity, hypertension, or diabetes, i.e., three risk factors linked to greater severity of SARS-CoV-2 infection [28].
A retrospective observational study showed that infected subjects had a more severe vitamin D deficiency than control subjects [12]. Another retrospective study reported worse outcomes in the disease for those patients with vitamin deficiency [3]. Adequate status has been associated with a 15% reduction in severe cases among the elderly. Ilie et al. [29] suggested a possible correlation between vitamin D status and incidence and mortality because of COVID-19, as they found particularly low levels in the aging population of the countries which had been more affected by the pandemic.
Even though there is information and knowledge pointing to a prophylactic and/or therapeutic value of vitamin D against COVID-19, there is a lack of conclusive data, so no final assertion can be made yet. Further research and trials are required so as to completely demonstrate this hypothesis [19]. Nevertheless, it can be advisable for the general population to keep adequate levels of this vitamin, either by sun exposure, diet, or supplementation, especially in the most vulnerable groups. This would be an easy, cheap, and safe option that could potentially help reduce the burden generated by the pandemic. When it comes to supplementation, interventions needs to be controlled, as high doses may lead to the appearance of hypercalcemia [11][28].
This entry is adapted from the peer-reviewed paper 10.3390/antiox11010005
References
- Sassi, F.; Tamone, C.; D’amelio, P. Vitamin D: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656.
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562.
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring links between Vitamin D deficiency and COVID-19. PLoS Pathog. 2020, 1, e1008874.
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.; Peroni, G.; Nichetti, M.; Perna, S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid. Based Complement. Altern. Med. 2018, 2018, 5813095.
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Holdford, P.; Thorton, C.; Whitaker, I. Could vitamins help in the fight against COVID-19? Nutrients 2020, 12, 2550.
- Zheng, S.X.; Yang, J.X.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parech, D.; Gao, F.; Trickett, D.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 1, 177.
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.; Donis, R. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333.
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270.
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchingset, N.; et al. MECHANISMS in ENDOCRINOLOGY Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147.
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 1, 236.
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of Vitamin D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. BioMed Cent. 2020, 18, 1–12.
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: Correlation with susceptibility and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9721–9738.
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92.
- Ali, N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public 2020, 13, 1373–1380.
- Koduah, P.; Paul, F.; Dörr, J.M. Vitamin D in the prevention, prediction and treatment of neurodegenerative and neuroinflammatory diseases. EPMA J. 2017, 8, 313–325.
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Pytel, V.; Montero, P.; Matías-Guiu, J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020, 10, e01498.
- Yancy, C.W. COVID-19 and African Americans. JAMA J. Am. Med Assoc. 2020, 323, 1891–1892.
- Grant, W.B.; Giovannucci, E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinology 2009, 1, 215–219.
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhatoa, H. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988.
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437.
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The inflammation link and the role of nutrition in potential mitigation. Nutrients 2020, 12, 1466.
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, G.D.; Ginde, A. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583.
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020.
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive effects of Vitamin D on seasonal influenza a in infants: A multicenter, randomized, open, controlled clinical trial. Pediatr. Infect Dis. J. 2018, 37, 749–754.
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260.
- Gruber-Bzura, B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419.
- Lee, M.D.; Lin, C.H.; Lei, W.T.; Chang, H.Y.; Lee, H.C.; Yeung, C.Y.; Chiu, N.; Chi, H.; Liu, J.; Hsu, R.; et al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients 2018, 10, 409.
- Galmés, S.; Serra, F.; Palou, A. Current state of evidence: Influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework. Nutrients 2020, 12, 2738.
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198.
This entry is offline, you can click here to edit this entry!
