Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Bone joint replacement is a major approach for restoring the functionalities of human joints caused by bone traumas or some chronic bone diseases. The structural design of the implant is crucial because the performance of the implant relies heavily on its geometry and microarchitecture. In addition, the optimization of the microstructure of bone implants also has an important impact on its performance. The additive manufacturing technique has enabled the production of bone joint replacements with more complex internal structures, which makes the design process more convenient.
- additive manufacturing
- numerical evaluation
- bionic design
1. Introduction
Bone trauma is a serious disease affecting the whole population worldwide. In many instances of bone trauma, especially those occurring in elderly people, bone joint replacement surgery has to be performed. Among these surgeries, hip and knee joint replacements are very common. According to statistical data, more than one million total hip replacements are performed each year in the world. Nowadays, people’s eating habits are very bad for bone health. For example, an excessive intake of salt will cause the loss of calcium, which in turn affects the health of bones. Patients suffering from joint pain and undergoing total joint arthroplasties, including total hip arthroplasties (THA), are getting younger and younger [2]. Due to these high demands, many different bone joint replacement products have been designed and relevant worldwide companies have become well established, e.g., DePuy, Johnson & Johnson, Smith & Nephew. The installation of joint replacements has enabled the restoration of human joint movement and, therefore, the daily activities of the human. However, there are still many issues related to the current joint replacements. For example, the wear and the micro-motion at the interface of the joint are still unsolved challenges, which cause the early failure and loosening of the joint replacement products [3]. The dislocation of the femur head is another common issue related to joint replacement. The design of the shape and microstructure of the joint replacements is one effective approach to solve these challenges because the shape and microstructure of the replacements can alter the load distribution and consequently the issues surrounding wear and head dislocation might be relieved [4].
2. The Design of the Bone Joint Replacement
The design of a bone joint replacement plays an important role in improving the performance of the joint replacement. The design objectives, the design variables, and the design constraints are the three key elements in the design. Ideally, the bone implant should have similar hierarchical configurations on multiple scales. Besides, the implant should possess properties similar to the host bone to match the mechanical performance. Therefore, the implant should possess both adequate stiffness to resist the physical loading and sufficient permeability since the transportation of cells requires the flow of blood through the implants [5].
A high stem stiffness means that most of the load is transferred from the prosthetic head to the distal femur by the stem itself. So, the bone tissue load in the epiphyseal region of the proximal femur is significantly lower than the physiological load. This effect is called ‘stress shielding’. Therefore, a structure with proper stiffness should be selected as the implant to prevent the stress-shielding effect caused by high stiffness [6]. Ścigała et al. [6] used internal lattice structures to reduce the stiffness of the hip endoprosthesis. They designed new structures and used the finite element method to analyze the stiffness of the implants. Their results showed that the use of inner lattice structures reduced implant stiffness and therefore potentially avoided the stress-shielding effect (Figure 1).
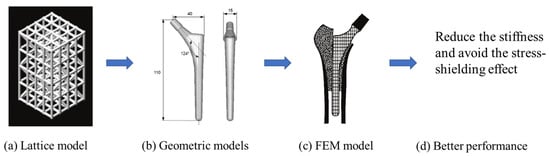
Figure 1. An example of obtaining better performance by designing a new structure. Adapted from reference [6].
Abdellah et al. [9] introduced a novel methodology to realistically design cemented hip prostheses by controlling the size of the implant cross sections, and they minimized Young’s modulus in this way. Thus, the stiffness is minimized to avoid stress shielding. However, further in vivo experiments need to be conducted to verify this conclusion. The stiffness of the structure has an important influence on its mechanical and biological properties. Thus, the stiffest design method is an effective optimization method. The stiffest design method generally comprises the optimization of size, shape, and topology. Nowak et al. [10,11] have conducted a lot of research on the stiffest design through mathematical models. In their work, the main assumption is that there is a constant strain energy density on the structural surface.
3. The Manufacturing of the Bone Joint Replacement
The manufacturing of bone joint replacements is closely related to the design of the joint replacements. When designing a joint replacement, the manufacturing processing, constraints, etc., should be taken into account. Otherwise, the designed products may not be producible. Therefore, the design of the joint replacement is largely influenced by the manufacturing techniques. The commonly used manufacturing techniques for producing bone joint replacements can be classified into the following groups: First, traditional manufacturing methods, such as rapid prototyping (RP) and Computer Numerical Control (CNC) [35]. Second, the emerging AM method. The RP approach is widely used in engineering for the quick fabrication of geometrically complex concepts. First, the 3D model of the implants should be constructed and sliced. Then, the computer controls the materials to be deposited on the operating platform to manufacture the bone implants [36]. Regarding the CNC method, the milling strategy and types of tools must be properly selected to achieve maximum accuracy in the resulting physical implant. Marcin et al. [37] presented clinical and technical information on temporomandibular joint replacements, where custom-made implants were manufactured using two different techniques: CNC and direct metal laser sintering (DMLS). In recent years, the emergence and application of the AM technique have enabled the production of bone joint replacements with more complex internal structures.
The AM is an emerging technique, which enables the production of nonhomogeneous and irregular structures. Among the various AM techniques, selective laser sintering (SLS), selective laser melting (SLM), electron beam melting (EBM), and binder jetting (BJ) have been successfully used to produce porous bone implants, such as knee implants. The selection of a repeatable and reliable manufacturing method is essential in manufacturing the design. Several factors can influence the selection of the manufacturing method, including the quantities required, the desired surface finish, the cleaning required post-fabrication, the risk of contamination during manufacturing, packaging, and sterilization [38].
4. The Evaluation of the Performance of the Bone Joint Replacement
The in silico, in vitro, and in vivo testing approaches are the three main types of method used to evaluate the performance of joint replacements.
The in silico method, also called the numerical method, uses the computational models to numerically evaluate the performance of a joint replacement. Once it is developed and validated, it can be efficiently used to evaluate the replacement’s performance. The numerical models developed in the literature can be classified into three groups. First, the musculoskeletal models are used. The musculoskeletal models are the models using rigid bones, muscles lines, etc., to simulate the activities of the human body. The models can be used to quickly evaluate the influence of the geometry of the replacements on the performance during daily activities, such as stair climbing, squatting, etc. Navacchia et al. [44] proposed a solution to address the inevitable tradeoff between computational cost and model detail in musculoskeletal simulations. However, because the musculoskeletal models are simplified as rigid models, detailed information regarding the stress and strain distribution within cannot be obtained, so the wear mechanism cannot be fully explored. To tackle these issues, the second approach, i.e., the coupled musculoskeletal-FE model, is used. The musculoskeletal-FE model uses rigid segments to represent the human body and uses the FE model to represent the part that is of great interest. The boundary conditions are passed from the musculoskeletal model to the FE model so that the FE model can be used to simulate different stages of daily activities. The advantage of this method is that both the overall and local behaviors in the joint replacement and the human tissues can be evaluated. However, defining the appropriate boundary conditions in the FE model is a challenging issue. The musculoskeletal model is a simplified model, and thus the boundary conditions obtained from it may not be accurate and appropriate. To solve this problem, FE models of the human body have been developed. FE models directly use the FE technique to simulate the daily activities of the human body. The challenge for this technique is to simulate the active contraction of the skeletal muscles, which is the driving force for musculoskeletal movements. The advantage of this approach is that the entire human segments are modeled using the deformable body and, consequently, the detailed mechanical information (stress, strain, etc.) can be obtained in every location within the model. However, because the detailed FE model is built, the computational cost is very large in the simulation of daily activities. Using this technique, Navacchia et al. [44] developed a computationally efficient muscle-force prediction strategy to track gait and chair rise experimental joint motion with a finite element of the musculoskeletal model of the lower limb.
This entry is adapted from the peer-reviewed paper 10.3390/ma15010153
This entry is offline, you can click here to edit this entry!
