Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Clinical Neurology
|
Respiratory System
Limbic encephalitis (LE) is an inflammatory disease of the brain, in which lesion is anatomically limited in structures of the limbic system. In some cases, LE can start with symptoms of limbic dysfunction with further involvement of other regions of the brain. Classic LE syndrome includes such symptoms as the development of personality disorders, depression, sleep disorders, epileptic seizures, hallucinations and cognitive disorders (short-term and long-term memory impairment). The information of clinical examination, electroencephalogram (EEG), magnetic resonance imaging (MRI) and cerebrospinal fluid studies (CSF) suggest the diagnosis of LE in most patients with Coronavirus Disease 2019 (COVID-19).
- limbic system
- limbic encephalitis
- COVID-19
- neurological complication
1. History of Limbic Encephalitis
Limbic encephalitis (LE) was first described in 1960 by Brierley et al. [1]. Later, in 1968, Corsellis et al. suggested the term “Limbic Encephalitis” [2]. For the next few decades, LE was considered a rare autoimmune disease strictly associated with cancer [3]. However, the development of neuroimaging and antineuronal antibodies detection methods demonstrated that LE can be divided in to two main variants: the first variant is LE, associated with paraneoplastic antibodies or cancer (typical LE), and the second variant is LE, without paraneoplastic antibodies or cancer (atypical LE) [4][5]. Studies in the following years showed that LE can be associated with variative antigen localization and different antibodies [6]. Other studies showed that most patients with LE are found to have antibodies to neuronal cell-surface antigens [7]. These antigens can be expressed in different regions of the central nervous system (CNS), and the limbic system (especially hippocampal formation) is known as one of the common localizations, followed by the cerebellum.
2. Para-Infectious Variants of Limbic Encephalitis
Alongside para-neoplastic LE, para-infectious LE [9] should be distinguished. It is primarily associated with production of antibodies to Herpesviridae family viruses, especially antibodies to Herpes simplex virus-1 (HSV-1). However, other members of this family (Varicella zoster, Cytomegalovirus, etc.) are almost never associated with neuroimaging changes typical to LE. An exception to this is Human herpesvirus 6 (HHV-6), which is considered as a possible cause of LE symptoms in patients with immunosuppression [6]. Due to chronic persistence of HSV-1 and HHV-6 in CNS, the pathological process in para-infectious LE is characterized not only by direct virus invasion but also by the production of specific autoantibodies, produced as result of cross presentation mechanisms [9].
Since 2019, increasing interest and attention have being given to the features of clinical presentation of COVID-19, caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). At this time, there is growing data, confirming the direct impact of SARS-CoV-2 on the limbic system of the brain and its immune-mediated damage [10][11][12]. According to the neuroimaging data, pathologic changes of the limbic system associated with COVID-19 can be both unilateral or bilateral (more common). Those changes are frequently found bilateral in the olfactory cortex, hippocampal formation, insula, right cingulate gyrus, left Rolandic operculum and left Heschl’s gyrus. Studies showed the correlation between neuroimaging findings and clinical manifestations, typical to LE associated with SARS-CoV-2. Thus, neuroimaging changes in hippocampal formation, right cingulate gyrus, left Rolandic operculum, and left Heschl’s gyrus correlated with cognitive disorders (memory impairment), while odor perception impairment (central hyposmia or anosmia) correlated with pathologic changes in mediobasal region of the temporal lobe (hippocampus) and the right cingulate gyrus [10]. In addition, the possible damage to the limbic system is testified by a number of case reports describing COVID-19 as a possible factor, provoking interictal and ictal epileptiform activity in temporal localization according to EEG, including seizures as the onset of CNS damage in children and adult patients with COVID-19 [13][14][15]. In addition, there are a number of studies describing COVID-19 patients with the development of such symptoms as cognitive disorders, personality disorders, anxiety-depressive disorders and, sometimes, aggressive behavior with psychosis [16][17]. This combined gives strong evidence of possible LE associated with SARS-CoV-2. Interestingly, these symptoms, typical for LE, have been described not only in patients in the acute phase of COVID-19 but also in the late recovery period [18][19].
Furthermore, cases of LE associated with the asymptomatic course of COVID-19 have been described. These cases show that neurological manifestations of COVID-19 are not always a reflection of the severe course of the disease. Because of that, an additional examination to exclude COVID-19 in patients with symptoms of limbic dysfunction may be considerable [20]. Due to the absence of viral RNA or specific antibodies in cerebrospinal fluid (CSF), it might be the case that damage of the limbic system in COVID-19 patients is primarily associated with the autoimmune response, induced by SARS-CoV-2. Therefore, the nature of this pathological process may be described as para-infectious, and high-field Magnetic Resonance Imaging (MRI) of limbic structures can be considered as an important method of intravital diagnosis of para-infectious LE associated with SARS-CoV-2 [20][21].
Given all this, it is reasonable to expect a SARS-CoV-2-associated LE to become a new multidisciplinary challenge in neurology and psychiatry practice. In addition, it seems to be the case that with the spread of the COVID-19 pandemic, the frequency of para-infectious LE associated with SARS-CoV-2 may exceed the frequency of LE associated with Herpesviruses, which makes this a topic of high concern requiring more studies.
Neuroanatomy of Limbic System and COVID-19
According to the data of previous studies, SARS-CoV-2 can be the cause of demyelinating processes, neurodegeneration and other changes associated with brain aging and neurodegenerative diseases [22]. However, only few clinical cases and thematic reviews describe the features of neurological changes in the acute phase of COVID-19, while the long-term consequences in COVID-19 patients are even less described [23].
Brain lesions caused by SARS-CoV-2 are variative. Among all possible targets, the involvement of the limbic system and limbic dysfunction caused by COVID-19 seems to be the most important one. The reason of such importance is explained by the fact that limbic dysfunction can cause not only personality disorders and behavioral changes (including schizoaffective disorders) but also lead to severe social disadaptation as a result. In the following section, we will discuss structures of the limbic system, which are primarily affected in COVID-19 patients (Table 1; Figure 1).
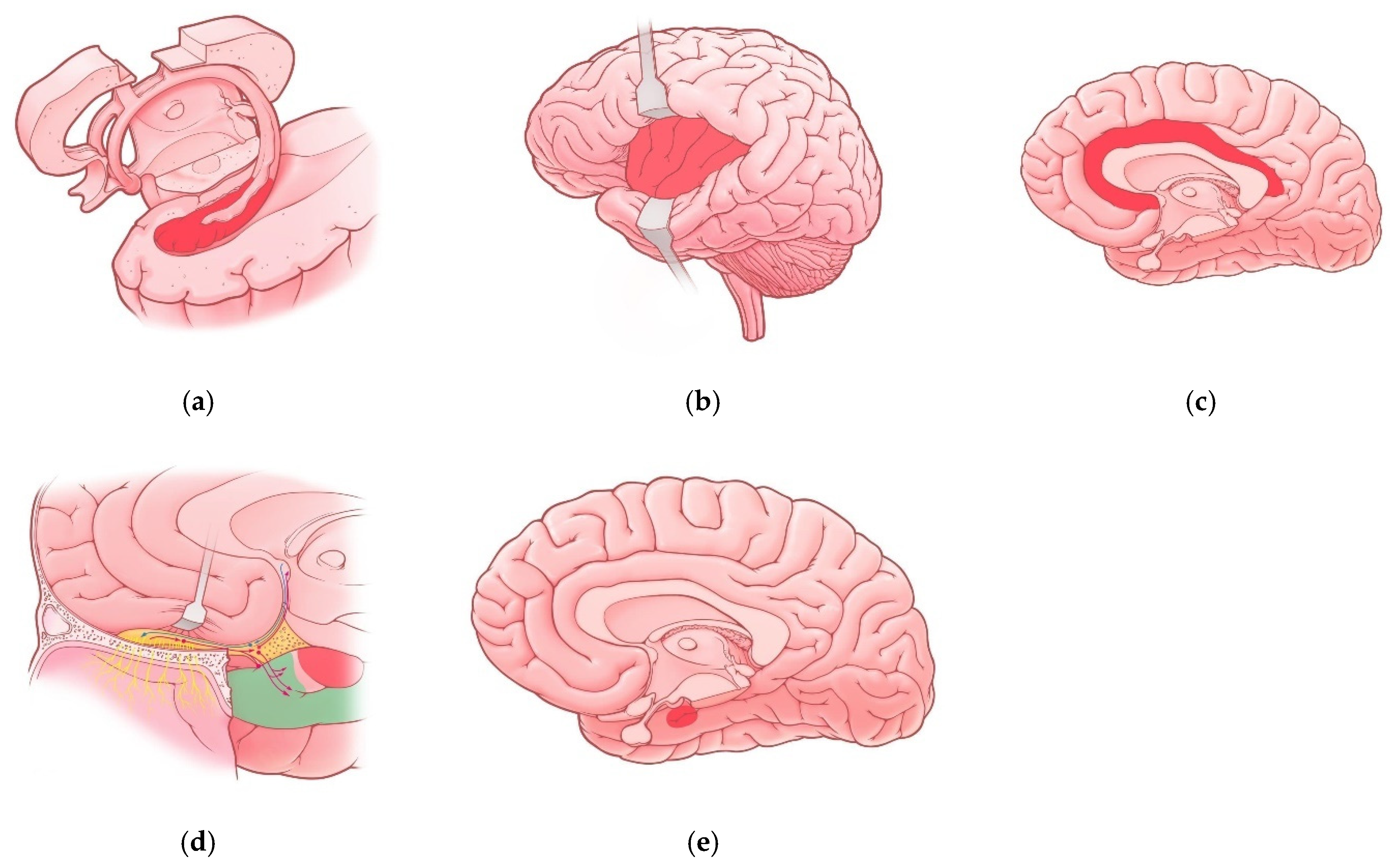
Figure 1. Regions of the limbic system affected in COVID-19 patients: (a) Hippocampus; (b) insula; (c) cingulate gyrus; (d) olfactory cortex—red-colored region represents uncus and green-colored region represents parahippocampal gyrus, red and blue lines represent olfactory pathways; (e) Amygdala (projection).
The hippocampus (hippocampal formation) is a complex structure of the brain located in the mesial region of the temporal lobe. The hippocampus plays a significant role in learning processes and memory. In addition, it is involved in the development of emotional and behavioral reactions and spatial orientation. Lesions of this region can lead to memory impairment, decreased learning ability, anxiety-depressive disorders, schizoaffective disorders and seizure onset [24]. According to studies, COVID-19-associated hippocampal lesions can feature with focal ictal and interictal epileptiform activity, mnestic process disturbances, impairment of episodic memory and consciousness impairment [10][25].
The amygdala (amygdaloid bodies) is a structure of the brain, represented by a nuclei formation, which is located in the anteromedial region of the temporal lobes. Functions of this structure include the development of adaptive reactions to emotionally significant events (especially threatening one), involvement in memory processes, face recognition [26][27] and also in olfactory perception [28]. Despite the available neuroimaging data showing changes in the amygdala of patients with COVID-19, the corresponding clinical features are still not characterized enough. However, some authors noticed a correlation between neuroimaging changes in the amygdala and possible vulnerability to depressive disorders in COVID-19 patients [29].
The insula is a region of the cerebral cortex that lies deep in the lateral sulcus. Due to numerous connections with other brain regions and structures, the insula takes part in variative processes: gustatory stimuli analysis, development of emotional reactions, empathy, evaluation of risk, attention control (recognition of a “new” stimuli), interoception, autonomic nervous system regulation, involvement in vestibular, auditory stimuli analysis and pain perception [30]. Damage to the insula can cause the impairment of gustatory perception (dysgeusia and ageusia), changes in emotional behavior (apathy, increased anxiety, decreased empathy) and memory impairment [31]. As for features accompanying changes in this region in COVID-19 patients, some case reports describe possible courses presenting disturbances in pain perception and interoception [32].
The cingulate gyrus is a part of the limbic system, known to be responsible for the development and processing of emotions, education and involved in memory processes. Pathological changes in the cingulate gyrus can lead to the development of depressive disorders, personality disorders and schizoaffective disorders (including psychosis) [33]. The involvement of this region is frequently described in patients with COVID-19, manifesting as the development of anxiety and depressive disorders, confusion and attention and short-term memory disturbances [10][23].
The olfactory cortex is a group of structures in the brain, including the olfactory nucleus, the olfactory bulb, the frontal and temporal parts of piriform cortex, partially the amygdala, the entorhinal cortex and the parahippocampal gyrus. Functionally, this region is responsible not only for the analysis of olfactory stimuli but also takes part in memory processes and the development of certain emotional or social reactions [34][35]. Pathological processes in the olfactory cortex can cause olfactory perception impairment, memory consolidation disturbances and trigger focal epileptiform activity-focal sensory or psychosensory epileptic seizures (olfactory hallucinations) [35][36]. COVID-19 is known to induce the altered olfactory perception as both an acute or long-lasting symptom. Altered olfactory perception in COVID-19 patients is known to manifest as an acute symptom as well as a long-term persisting condition. Moreover, these alterations are variative: hyposmia—decreased perception of odors; anosmia—complete absence of odor perception; parosmia—distorted perception of common odors; and phantosmia—the sensation of smell in the absence of its source. Due to frequent negative characteristic of altered smell (malodorant, fetid smell), olfactory perception distortions can have a significant impact on quality of life in patients with COVID-19 [37][38][39].
Parosmia can be associated with central or peripheral lesions, caused by SARS-CoV-2, because COVID-19 can affect both the olfactory bulb and the cortical centers of the olfactory analisator [38]. In addition, paradoxical regrowth of new olfactory axons can prolong the parosmia in patients after COVID-19. However, studies suggest olfactory training as an effective method of improving olfactory function in patients with COVID-19-associated persistent parosmia [40].
In comparison, Araújo et al. (2021) suggest a death of olfactory neurons and neurodegeneration in the olfactory bulb to be a primary cause of persistent olfactory dysfunction in patients after COVID-19 [41]. Thus, studies of the brain using 18F-FDG-PET showed hypometabolism in the left orbitofrontal cortex, which correlated with olfactory bulb edema in MRI [37][38]. In addition, persistent anosmia can present as a single symptom in COVID-19 patients, therefore, it should be considered as a possible marker for this infection [42].
Table 1. Regions of the limbic system affected in COVID-19 patients.
| Region of Limbic System | Associated Manifestations in COVID-19 Patients | References |
|---|---|---|
| Hippocampus | Memory impairment Impaired Consciousness Seizures |
[10][25] |
| Insula | Pain perception disturbances Interoception disturbances |
[32] |
| Cingulate gyrus | Anxiety disorders Depressive disorders Cognitive impairments (brain fog) Impaired episodic memory Attention disturbances |
[23] |
| Olfactory cortex | Olfactory dysfunction (hyposmia, anosmia) |
[10][41] |
| Amygdala | Vulnerability to depressive disorders | [29] |
Damage of the olfactory cortex attributed to COVID-19 generally leads to olfactory disturbances (hyposmia and anosmia) [10], but the frequency and intensity of those symptoms are variative in patients, even if age and the severity of COVID-19 are comparable. Thus, the frequency of olfactory dysfunction in COVID-19 patients can range from 15% to 98% [43][44][45]. However, it is reasonable to mention that specifics of used methods of interviewing patients and information gathering can have significant influence, potentially causing this variety.
The real cause for this phenomenon is not fully understood yet. Perhaps hyposmia or anosmia persisting after COVID-19 can be considered as a marker of limbic dysfunction and LE in general. In the long-term perspective, persisting anosmia can have an impact on the quality of patients’ lives, also possibly accompanied with the development of depression [46][47], anxiety [48], problems with socialization [49][50], cognitive impairment [49][51][52] and anorexia with related complications [48].
Moreover, Pirker-Kees et al. (2021) demonstrated that olfactory dysfunction can be associated with cognitive impairment, even in patients with mild COVID-19 [53]. In addition, since previous studies [54] showed correlation between olfactory dysfunction and cognitive impairment with neuroimaging changes in the limbic system, altered olfactory perception presumably can be considered as marker of limbic system involvement in patients with mild and subclinical COVID-19.
3. Discussion
Given all this, para-infectious LE, associated with SARS-CoV-2, meets the definition of autoimmune LE according to Graus’ criteria [55]:
- (1) Subacute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status or psychiatric symptoms associated with limbic system involvement;
- (2) At least one of the following:
-
- -New focal CNS findings, seizures not explained by a previously known seizure disorder;
- -CSF pleocytosis (white blood cell counts of more than five cells per mm3);
- -MRI features suggestive of encephalitis;
- (3) Reasonable exclusion of alternative causes.
When all three criteria are met, a diagnosis of LE can be made.
Therefore, analyzed studies [10][21][23][25][32] show that para-infectious LE, associated with SARS-CoV-2, can be considered as an independent disease, which should be studied more because the possible consequences for patients and society are unclear, while the frequency and prognosis of this disease still need to be clarified. Interestingly, it is possible for para-infectious LE, associated with SARS-CoV-2 to occur in patients with respiratory system involvement, as well as in patients with no respiratory symptoms [10], including single cases of isolated attack on the limbic system [20]. In the majority of described clinical cases, the most common neurological symptoms of this disease were: cognitive impairment; focal and generalized tonic-clonic seizures, including the development of status epilepticus; neuropsychiatric symptoms (olfactory and visual hallucinations, short-term memory loss, anxiety-depressive disorders, socialization problems); and olfactory dysfunction [10][21][23][25][32]. The presence of ictal or (more common) interictal abnormal activity located in the temporal lobe in electroencephalography (EEG) should be considered as a significant factor [13]. According to MRI, damage of the limbic system in COVID-19, in most cases, was bilateral in the form of T2-weighted imaging (T2) and Fluid attenuated inversion recovery (FLAIR) hyperintensity. Moreover, contrasting with gadolinium increased the frequency of those findings [10][20].
MR-spectroscopy with evaluation markers such as peak of N-acetylaspartate (NAA), Choline (Cho), Creatine (Cr), Lactate (Lac), Glutamate–Glutamine complex (Glx) and Myoinositol (MI) can become a promising method for diagnosing neurodegenerative and neuroinflammatory processes in para-infectious LE associated with SARS-CoV-2 [56] (Table 2).
Table 2. Brain metabolites used for magnetic resonance spectroscopy in patients with limbic encephalitis [56].
| Metabolite | Commentary |
|---|---|
| N-acetylaspartate (NAA) Chemical Shift = 2.02 |
It is a derivative of amino acids synthesized in neurons and further transported along axons. A reliable marker of the viability of neurons, axons and dendrites. |
| Choline (Cho) Chemical Shift = 3.22 |
It is part of cell membranes and cholinergic synaptic endings of neurons and takes part in lipid metabolism. |
| Creatine (Cr) Chemical shift = 3.02; 3.94 |
The main marker of energy processes in astrocytes and neurons. It is formed during the conversion of the high-energy compound Adenosine triphosphate to Adenosine diphosphate. In the brain tissue, the signal intensity in many cases remains constant, even with pathological changes. |
| Lactate (Lac) Chemical Shift = 1.33 |
It is not detected in normal brain tissue. It is contained in the cerebrospinal fluid (0.9 mmol/L). Indicator of anaerobic glycolysis. |
| Glutamate-Glutamine Complex (Glx) Chemical shift = 2.1–2.5 |
Astrocyte marker and neurotoxin, respectively. Glutamine (2-aminopentanamide-5-ovic acid) is the 1 of the 20 standard amino acids that make up the protein. Excitatory neurotransmitter. |
| Myoinositol (MI) Chemical Shift = 3.56 |
Myelin degradation product. The concentration rises in multiple sclerosis and decreases in tumors. It rises in the center of the epileptogenic focus and decreases in the tissues adjacent to it. |
An interesting fact is that in most patients with SARS-CoV-2, serological tests on autoimmune panels, including autoantibodies against intracellular, synaptic and surface antigens previously described in LE of a different etiology, were negative [21]. According to this fact, some authors consider the development of SARS-CoV-2-associated para-infectious LE caused by secondary hyperinflammatory syndrome with a massive release of proinflammatory cytokines and chemokines in the limbic system of the brain [21][57][58]. Taken together, these facts explain the scientific and clinical interest in studying the role of pro-inflammatory cytokines, including interleukin-1-beta (IL-1), interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), in the development of para-infectious LE associated with SARS-CoV-2, as overexpression of these cytokines has been described in previous studies of drug resistant para-infectious temporal lobe epilepsy [59][60][61][62][63] and also in cognitive disorders including shot-term and long-term memory [64] and Alzheimer’s type dementia [65].
The overexpression of proinflammatory cytokines can be genetically determined [66], which can explain the high risk of developing para-infectious LE associated with SARS-CoV-2 in patients with an unfavorable genetic profile. In addition, the autoimmune mechanisms of LE associated with SARS-CoV-2 is unlikely to be associated with the production of antineuronal autoantibodies, provoked by the virus and possible molecular mimicry [21]. However, we should admit that the number of questions about the problem of LE associated with SARS-CoV-2 highly exceeds the number of answers we currently have.
4. Limitations
The publications that we have analyzed over the past two years show a small number of reports of clinical cases of SARS-CoV2-associated LE yet. However, the number of cases will increase with the growth of publications and research on this important problem of neurology and psychiatry. Currently, a study of the frequency of occurrence of this disease in Russia and in the world has been launched. The results of new studies may be analyzed in the future.
Probably, there are any other factors (age, gender, specific underlying pathological conditions, etc.) likely related to the involvement of the limbic system in patients with COVID-19 and post-COVID-19 syndrome. This is a very interesting and important question. We agree that the study of these factors (environmental, non-genetic and genetic) is of undoubted scientific and clinical interest. Other recent literature concerning autoimmune encephalitis and SARS-CoV2 are discussed, and the difference between SARS-CoV2-associated autoimmune encephalitis and LE is also very important [67]. However, this topic is currently insufficiently studied, despite its high relevance. We are studying these factors, but so far, the duration of the study is short, so today we cannot give a definite answer to this question.
There are summary ideas and the available knowledge about SARS-CoV2-associated LE in this entry paper. However, the requirements for the entry paper do not allow us to consider the proposed mechanisms of SARS-CoV2′s effects on the limbic system in more detail.
This entry paper demonstrates that the number of confirmed cases of SARS-CoV2-associated LE has been increasing over the past year. This may be due to a new strain of this virus or antigenic mimicry. In addition, the authors’ own clinical experience shows that in the last three months alone, four cases of this disease were identified. They all require separate reports.
The general limitations are that there are currently more questions about LE associated with SARS-CoV-2 than answers.
5. Conclusions
Concluding, we should mention that the number of publications dedicated to para-infectious LE associated with SARS-CoV-2 is currently limited. However, the high tropism of SARS-CoV-2 to the structures of the limbic system of the brain, with the possibility of isolated lesions of these areas in children and adults without respiratory symptoms of COVID-19, and the high number of new cases of this infection in population worldwide indicates the importance of this interdisciplinary problem in neurology and psychiatry. In addition, the development of an autoimmune process in the CNS is also possible after the respiratory symptoms was resolved, as the long-term consequence in patients with the pulmonary form of COVID-19. In patients with a high risk of developing para-infectious LE associated with SARS-CoV-2, the diagnostic algorithm in the acute and subacute periods should include analysis of CSF, EEG monitoring and brain MRI. In patients with asymptomatic or mild COVID-19 (without respiratory symptoms) and long-term consequences of COVID-19 (long-COVID-19), the diagnostic algorithm should include neuropsychological testing, brain MRI, EEG and serological testing (evaluating the pro-inflammatory cytokines and antibodies to SARS-CoV-2 IgG). This algorithm can help in the risk assessment and early diagnosis of para-infectious LE associated with SARS-CoV-2; moreover, it gives information for consideration in treatment planning. An important but controversial issue is the acceptability of revaccination against COVID-19 in patients with high risk of developing para-infectious LE or patients with this disease.
Future research on biomarkers for the risk of developing para-infectious LE associated with SARS-CoV-2 and its severity is necessary to expand our knowledge about this new immune-mediated CNS disease and its possible consequences for patients and society.
References
- Brierley, J.B.; Corsellis, J.A.N.; Hierons, R. Subacute encephalitis of later adult life. Mainly affecting the limbic areas. Brain 1960, 83, 357–368.
- Corsellis, J.A.; Goldgerg, G.J.; Norton, A.R. “Limbic encephalitis” and its association with carcinoma. Brain 1968, 91, 481–496.
- Bakhelt, A.M.; Kennedy, P.G.; Behan, P.O. Paraneoplastic limbic encephalitis: Clinico-pathological correletions. J. Neurol. Neurosurg. Psychiatry 1990, 53, 1084–1088.
- Gultekin, S.H.; Rosenfeld, M.R.; Voltz, R.; Eichen, J.; Posner, J.B.; Dalmau, J. Paraneoplastic limbic encephalitis: Neurological symptoms, immunological findings and tumor association in 50 patients. Brain 2000, 123, 1481–1494.
- Vitaliani, R.; Mason, W.; Ances, B.; Zwerdling, T.; Jiang, Z.; Dalmau, J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann. Neurol. 2005, 58, 594–604.
- Tüzün, E.; Dalmau, J. Limbic encephalitis and variants: Classification, diagnosis and treatment. Neurologist 2007, 13, 261–271.
- Wagner, J.; Witt, J.A.; Helmstaedter, C.; Malter, M.P.; Weber, B.; Elger, C.E. Automated volumetry of the mesiotemporal structures in antibody-associated limbic encephalitis. J. Neurol. Neurosurg. Psychiatry 2014, 86, 735–742.
- Shnayder, N.A.; Dmitrenko, D.V.; Dykno, Y.A.; Ezhikova, V.V. Paraneoplastic limbic encephalitis in neurological and oncological practice. Russ. J. Oncol. 2013, 1, 49–57.
- Shnayder, N.A.; Panina, Y.S.; Dmitrenko, D.V.; Krjzhanovskaya, S.V.; Molgachev, A.A. Parainfectious limbic encephalitis associated with herpes viridae viruses. Probl. Women Health 2014, 9, 58–69.
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients–An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484.
- Bridwell, R.; Long, B.; Gottlieb, M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020, 38, 1549.e3–1549.e7.
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120.
- Kurd, M.; Hashavya, S.; Benenson, S.; Gilboa, T. Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure 2021, 92, 89–93.
- Sohal, S.; Mansur, M. COVID-19 Presenting with Seizures. IDCases 2020, 20, e00782.
- Santos de Lima, F.; Issa, N.; Seibert, K.; Davis, J.; Wlodarski, R.; Klein, S.; El Ammar, F.; Wu, S.; Rose, S.; Warnke, P.; et al. Epileptiform activity and seizures in patients with COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 565–566.
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386.
- Troyer, E.A.; Kohn, J.N.; Hong, S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020, 87, 34–39.
- Carroll, E.; Neumann, H.; Aguero-Rosenfeld, M.E.; Lighter, J.; Czeisler, B.M.; Melmed, K.; Lewis, A. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia 2020, 61, e135–e139.
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020, 87, 18–22.
- Zambreanu, L.; Lightbody, S.; Bhandari, M.; Hoskote, C.; Kandil, H.; Houlihan, C.F.; Lunn, M.P. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1229–1230.
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F.; et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav. Immun. Health 2021, 12, 100–210.
- Dinakaran, D.; Manjunatha, N.; Naveen Kumar, C.; Suresh, B.M. Neuropsychiatric aspects of COVID-19 pandemic: A selective review. Asian J. Psychiatry 2020, 53, 102–188.
- Hugon, J.; Msika, E.F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2021, 18, 1–3.
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246.
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58.
- Adolphs, R.; Tranel, D.; Hamann, S.; Young, A.W.; Calder, A.J.; Phelps, E.A.; Anderson, A.; Lee, G.P.; Damasio, A.R. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia 1999, 37, 1111–1117.
- McGaugh, J.L. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004, 27, 1–28.
- Patin, A.; Pause, B.M. Human amygdala activations during nasal chemoreception. Neuropsychologia 2015, 78, 171–194.
- Zhang, S.; Cui, J.; Zhang, Z.; Wang, Y.; Liu, R.; Chen, X.; Feng, Y.; Zhou, J.; Zhou, Y.; Wang, G. Functional connectivity of amygdala subregions predicts vulnerability to depression following the COVID-19 pandemic. J. Affect. Disord. 2021, 297, 421–429.
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306.
- Di Stefano, V.; De Angelis, M.V.; Montemitro, C.; Russo, M.; Carrarini, C.; di Giannantonio, M.; Brighina, F.; Onofrj, M.; Werring, D.J.; Simister, R. Clinical presentation of strokes confined to the insula: A systematic review of literature. Neurol. Sci. 2021, 42, 1697–1704.
- Coen, M.; Kaiser, C.; Naimi, R.; Uginet, M.; Hentsch, L.; Serratrice, J.; Allali, G. Beyond silent hypoxemia: Does COVID-19 can blunt pain perception? Comment on The neuroinvasive potential of SARS CoV2 may play a role in the respiratory failure of COVID 19 patients. J. Med. Virol. 2021, 93, 1915–1916.
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain A J. Neurol. 2014, 137, 12–32.
- Zhou, G.; Lane, G.; Cooper, S.L.; Kahnt, T.; Zelano, C. Characterizing functional pathways of the human olfactory system. Elife 2019, 24, e47177.
- Takehara-Nishiuchi, K. Entorhinal cortex and consolidated memory. Neurosci. Res. 2014, 84, 27–33.
- Vaughan, D.N.; Jackson, G.D. The piriform cortex and human focal epilepsy. Front. Neurol. 2014, 5, 259.
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833.
- Yousefi-Koma, A.; Haseli, S.; Bakhshayeshkaram, M.; Raad, N.; Karimi-Galougahi, M. Multimodality Imaging With PET/CT and MRI Reveals Hypometabolism in Tertiary Olfactory Cortex in Parosmia of COVID-19. Acad. Radiol. 2021, 28, 749–751.
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021, 20, 753–761.
- Makaronidis, J.; Firman, C.; Magee, C.G.; Mok, J.; Balogun, N.; Lechner, M.; Carnemolla, A.; Batterham, R.L. Distorted chemosensory perception and female sex associate with persistent smell and/or taste loss in people with SARS-CoV-2 antibodies: A community based cohort study investigating clinical course and resolution of acute smell and/or taste loss in people. BMC Infect. Dis. 2021, 21, 221.
- Araújo, L.; Arata, V.; Figueiredo, R.G. Olfactory Disorders in Post-Acute COVID-19 Syndrome. Sinusitis 2021, 5, 116–122.
- Mehraeen, E.; Behnezhad, F.; Salehi, M.A.; Noori, T.; Harandi, H.; SeyedAlinaghi, S. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence. Eur. Arch. Otorhinolaryngol. 2021, 278, 307–312.
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinology 2020, 58, 430–436.
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 13, 594.
- Costa, K.V.T.D.; Carnaúba, A.T.L.; Rocha, K.W.; Andrade, K.C.L.; Ferreira, S.M.S.; Menezes, P.L. Olfactory and taste disorders in COVID-19: A systematic review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792.
- Hur, K.; Choi, J.S.; Zheng, M.; Shen, J.; Wrobel, B. Association of alterations in smell and taste with depression in older adults. Laryngoscope Investig. Otolaryngol. 2018, 3, 94–99.
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The Association Between Olfaction and Depression: A Systematic Review. Chem. Senses 2016, 41, 479–486.
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life–an updated review. Chem. Senses 2014, 39, 185–194.
- Valsamidis, K.; Printza, A.; Constantinidis, J.; Triaridis, S. The Impact of Olfactory Dysfunction on the Psychological Status and Quality of Life of Patients with Nasal Obstruction and Septal Deviation. Int. Arch. Otorhinolaryngol. 2020, 24, e237–e246.
- Schablitzky, S.; Pause, B.M. Sadness might isolate you in a non-smelling world: Olfactory perception and depression. Front. Psychol. 2014, 5, 45.
- Nordin, S.; Brämerson, A. Complaints of olfactory disorders: Epidemiology, assessment and clinical implications. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 10–15.
- Ahmedy, F.; Mazlan, M.; Danaee, M.; Abu Bakar, M.Z. Post-traumatic brain injury olfactory dysfunction: Factors influencing quality of life. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1343–1351.
- Pirker-Kees, A.; Platho-Elwischger, K.; Hafner, S.; Redlich, K.; Baumgartner, C. Hyposmia Is Associated with Reduced Cognitive Function in COVID-19: First Preliminary Results. Dement. Geriatr. Cogn. Disord. 2021, 50, 68–73.
- Dintica, C.S.; Marseglia, A.; Rizzuto, D.; Wang, R.; Seubert, J.; Arfanakis, K.; Bennett, D.A.; Xu, W. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 2019, 92, e700–e709.
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404.
- Solomatova, E.S.; Shnayder, N.A.; Molgachev, A.A.; Dmitrenko, D.V.; Strotskaya, I.G. Magnetic resonance spectroscopy of the brain in the diagnosis of temporal lobe epilepsy. Neurol. Neuropsychiatry Psychosom. 2018, 10, 51–55.
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034.
- Perrin, P.; Collongues, N.; Baloglu, S.; Bedo, D.; Bassand, X.; Lavaux, T.; Gautier-Vargas, G.; Keller, N.; Kremer, S.; Fafi-Kremer, S.; et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021, 28, 248–258.
- Popova, T.E.; Shnayder, N.A.; Petrova, M.M.; Nikolaeva, T.Y.; Kantimirova, E.A.; Isaeva, N.V.; Shnayder, V.A.; Panina, Y.S.; Dyuzhakova, A.V.; Dyuzhakov, S.K. Herpesvirus-associated central and peripheral nervous system involvement: Two clinical cases. Neurol. Neuropsychiatry Psychosom. 2015, 7, 28–34.
- Shnayder, N.A.; Panina, Y.S.; Popova, T.E. A clinical case of pseudotumorous chronic parainfectious limbic encephalitis. Neurol. Neuropsychiatry Psychosom. 2014, 6, 49–54.
- Shnayder, N.A.; Kamzalakova, N.I.; Krijanovskaya, S.V.; Panina, Y.S. Perceived ways of diagnostic help optimisation to patients with symptomatic epilepsy on chronic herpesvirus encephalitis. Epilepsy Paroxysmal Cond. 2015, 7, 6–17.
- Lorigados Pedre, L.; Morales Chacón, L.M.; Pavón Fuentes, N.; Robinson Agramonte, M.L.A.; Serrano Sánchez, T.; Cruz-Xenes, R.M.; Díaz Hung, M.L.; Estupiñán Díaz, B.; Báez Martín, M.M.; Orozco-Suárez, S. Follow-Up of Peripheral IL-1β and IL-6 and Relation with Apoptotic Death in Drug-Resistant Temporal Lobe Epilepsy Patients Submitted to Surgery. Behav. Sci. 2018, 8, 21.
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 277–286.
- Hansen, N. Long-term memory dysfunction in limbic encephalitis. Front. Neurol. 2019, 10, 330.
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050.
- Panina, Y.S.; Dmitrenko, D.V.; Shnayder, N.A.; Egorova, E.V.; Usoltseva, A.A. Association of carriers of single nucleotide polymorphisms rs1143634 and rs16944 in gene Il-1B and rs6265 in gene BDNF with temporal lobe epilepsy. Neurol. Neuropsychiatry Psychosom. 2019, 11, 46–51.
- Sanchez, C.V.; Theel, E.; Binnicker, M.; Toledano, M.; McKeon, A. Autoimmune encephalitis after SARS-CoV-2 infection: Case frequency, findings, and outcomes. Neurology 2021, 97, e2262–e2268.
This entry is offline, you can click here to edit this entry!
