The dynamics of forensic insects can operate at many spatial scales, manifest in different spatial patterns, and be attributed to multiple different causes. This highlights the importance for forensic entomology to consider spatial effects despite its neglect to date. Forensic entomology has much to benefit from the use of spatial statistics because many important questions, both at the fundamental and practical levels, require a spatial solution.
- autocorrelation
- blow flies
- Calliphoridae
- forensic sciences
- forensic entomology
- spatial statistics
1. Introduction
Forensic entomology is the scientific discipline that uses biological and ecological knowledge of insect species to obtain information in criminal investigations. It can allow to deduce clues about the circumstances of a death based on the community of insects assembling on this ephemeral resource that is the remains of large vertebrates. To date, forensic entomologists have concentrated most of their efforts on documenting the effects of time and abiotic parameters on insect occurrence and development [1][2]. In contrast, little attention has been given to spatial and scale effects [3][4][5][6][7][8] despite their pervasive influence on most of the biotic and abiotic parameters affecting insect occurrence and development. In this text, space refers to the place where the organisms involved in decomposition interact with each other and with the abiotic environment [9]. In turn, scale here refers to the size (i.e., the extent) of a study, although scale also technically involves grain, which may be defined as the dimension of sampling units (e.g., 1 ha, 1 Km² , 100 Km²) [10].
Blow flies (Diptera: Calliphoridae) are often studied and used in a forensic context because they colonize cadavers rapidly and in large numbers [1] (Figure 1). This is due to their remarkable dispersal capabilities and effectiveness at detecting and tracking volatile emissions from cadavers and carcasses [1]. Thus, blow fly species would be expected to be evenly distributed in space in response to carrion rather than in response to landscape configuration. However, many studies have reported that blow flies have habitat associations that are species-specific [7][11][12], implying the presence of spatial distribution processes other than simple resource effects. Such processes can be uncovered by investigating the presence of spatial effects acting on the distribution of blowflies.

Figure 1. Blow flies on the remains of a large vertebrate (Photo by Gaétan Moreau).
2. Spatial Statistics
Spatial statistics provide rigorous techniques for drawing inferences about spatially distributed objects. In the case of forensic entomology, a good example of spatially distributed objects would be a series of cadavers or carcasses in a field. This type of data needs to be analyzed using spatial statistics such as Inverse Distance Weighting (IDW), semivariograms and crossvariograms (Figure 2). The former is an interpolation (i.e., estimation of unknown data according to and within range of known data) technique using georeferenced data and relative distances to compute smoothed values throughout an area (Figure 2a). The basis of this analysis is based on Tobler’s famous quote that “Everything is related to everything else, but near things are more related than distant things” [13]. Semivariograms evaluate statistical dependency between data points (Figure 2b) while crossvariograms evaluate statistical dependency between two sets of spatially distributed objects (Figure 2c). Statistical dependency may also be referred to as autocorrelation/anticorrelation in statistical terms and as aggregation/segregation in ecological terms. Other commonly used spatial analyses that are not discussed in this text include kriging, geographically weighted regressions, point pattern analyses, etc.
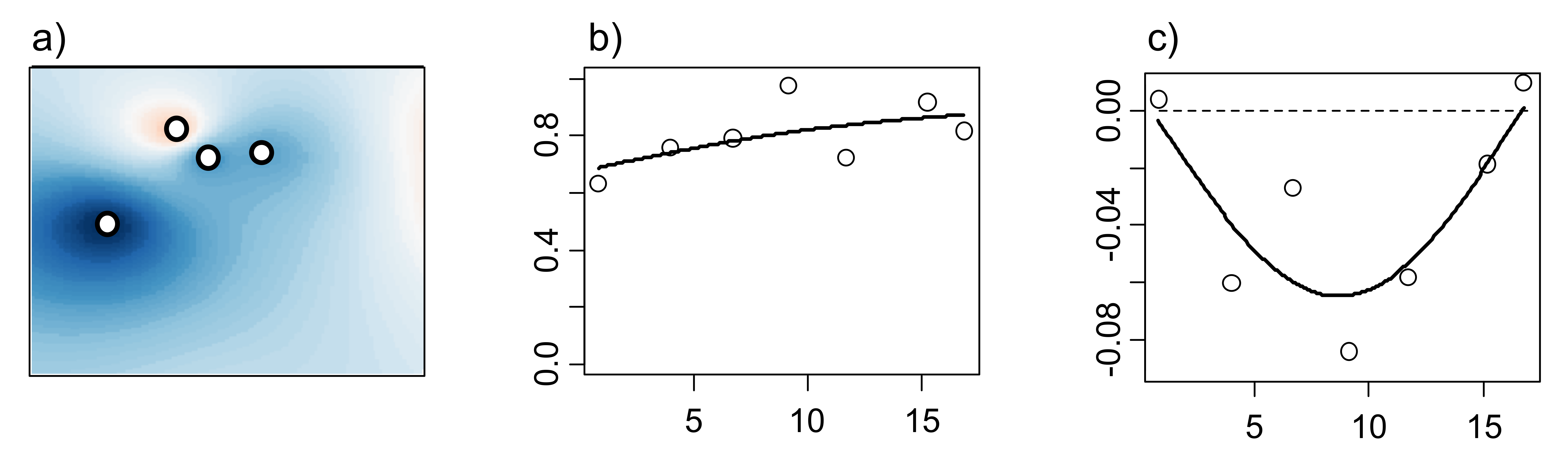
Figure 2. Inverse Distance Weighting (a) showing Phormia regina density in space (blue and red areas correspond to higher and lower densities, respectively), semivariogram of P. regina (b) and crossvariogram between P. regina and Calliphora livida (c).
Forensic entomology has much to gain from the implementation of statistics concerned with spatial and scale effects [14] as failure to consider spatial effects can lead to a cascade of errors. For example, local-scale effects can occur in body farms or small field sites due to heterogeneity in field conditions [15][16], microclimate [17], and movement between cadavers/carcasses of organisms that can synchronize carcass dynamics (i.e., forensic species of interest, predators, parasitoids, scavengers; [18][19]). Large-scale spatial effects can occur due to responses to climatic conditions [20], metapopulation and metacommunity dynamics, source-sink dynamics [21] and habitat specialization of forensic species of interest [22]. Many other situations can also create spatial patterns and failure to consider spatial layers can lead, for example, to underestimation of the influence of spatial dynamics on processes, to misattribution of spatial effects to other sources of variation [23], to inaccurate mechanistic inference, to erroneous projections from the data, such as when estimating the postmortem interval, etc.
3. Spatial Dynamics of Blow Flies
Commercial traps modified to recover blow flies of forensic interest were baited with minced pork liver [24]. The traps were set up within and on the outskirts of Moncton, Dieppe and Riverview in New Brunswick, Canada. Three linear transects were traced from urban areas to three different cardinal points and along each transect, a trap was placed in an urban area, a periurban area and a forest area (Figure 3), for a total of 9 traps. These traps allowed for the collection of several thousand blow flies. The regional distribution of the most abundant species was analyzed using the spatial statistical methods discussed above.
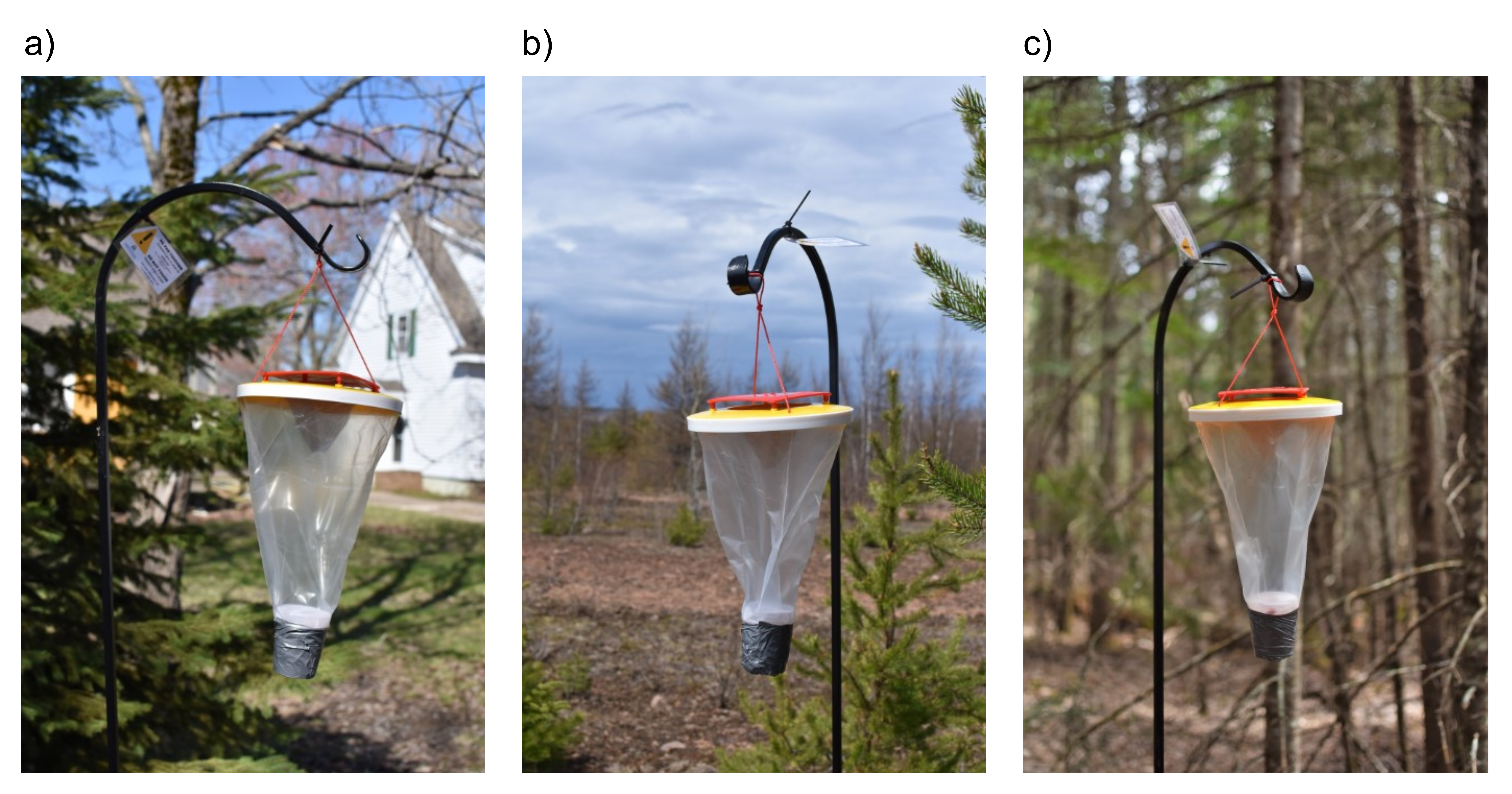
Figure 3. Modified traps in an urban (a), a periurban (b) and a forest area (c).
Regional distribution patterns of blow flies detected using spatial statistics were either novel or reinforced inferences about species-environment associations. For example, Lucilia sericata, a blow fly species capable of inhabiting urban or anthropized ecosystems [11][25], exhibited aggregated distributions concentrated in urbanized areas. Another abundant species in this genus, Lucilia illustris, exhibited local aggregation patterns, but these patterns did not match the landscape configuration. As aggregation patterns have been previously detected in Lucilia [4], this suggests that such a mechanism may play a particularly important role in the spatial structure of this genus. One species of particular importance in forensic entomology, Phormia regina, did not show a local aggregation pattern, but did show strong autocorrelation with L. illustris. We speculate that these synchronized spatial dynamics can be explained by the fragmentation of the resource, the generalism of both species, and their strong competitiveness [24][26][27][28]. The less pronounced spatial aggregation of P. regina compared to L. illustris may be explained by the greater ecological plasticity of the former and/or by the fact that it is a late colonizer of carrion in the study area compared to the latter [28][29]. The last species examined, Calliphora livida, showed a preference for forest areas as well as an anticorrelation with all other species. This interspecific segregation could be explained by the preference of this species for colder climatic conditions than late summer [24][30], which would lead the species to favor habitats that are not as optimal for its competitors. This demonstrates how seasonality and biotic interactions such as competition can play a role in the spatial partitioning of habitat between species [31].
4. Contribution of Spatial Statistics to Forensic Science
Most forensic studies examining species distribution report qualitative associations of species to environmental variables that are implicitly spatial (e.g., habitat, altitude; [7][11][12]). However, the local density of a species can vary within and among habitats [6], calling into question the validity of these qualitative associations and of studies that do not account for spatial heterogeneity. Indeed, the spatial analyses discussed above have indicated that processes other than simple environmental/habitat associations govern the regional distribution of all the blow flies species studied but L. sericata. Spatial analyses can thus uncover mechanisms not typically explored in forensic entomology. However, these analyses are not an end but rather a means to generate new hypotheses and better understand the behavior of a system. Indeed, spatial analyses allow visualization of spatial structure, which is probably more important in the applied context of forensic entomology than the reasons for that structure.
This study offers little information on small-scale spatial analysis because regional data were used to study blow flies distribution patterns. In the experimental context of forensic entomology, small-scale effects are likely to bias results, especially if cadavers/carcasses or forensic insect traps are located in close proximity to each other. Biotic interactions (e.g., competition, predation), which may be the primary drivers of processes at this scale [32], may also lead to species shifts along microhabitat quality gradients, causing interspecific aggregation/segregation patterns [33]. Such local spatial effects could even generate a consistent pattern of insect succession that would not be present in isolated carcasses and would therefore be an artefact. Thus, the need for small-scale spatial studies of autocorrelation in forensic entomology is stressed. Spatial effects also extend beyond the regional scale. On a larger scale, it is possible to examine whether insect species assemblages are governed by the same factors in different geographic areas. When variables are chosen wisely and space is well considered, multi-regional studies can also be more powerful in identifying causal relationships. Forensic entomology would certainly benefit from such ecological studies, which would provide a better understanding of species-environment relationships and the large-scale processes that influence the distribution of species of forensic interest.
5. Recommendations to Forensic Entomologists
The results of this study show that the spatial dynamics of blow flies are different for each species and are determined by more than simple habitat associations. Thus, some recommendations are offered to forensic entomologists and experts in related fields:
- The thumb rule of using a minimum distance of 50 m between cadavers or carcasses (e.g., [3][8][34][35]) to achieve some independence amongst experimental units needs to be re-evaluated. This could be done with approaches documenting distance-dependent autocorrelation while accounting for time dependence associated with repeated sampling (e.g., spatio-temporal semivariograms). Although the 50 m distance limits larval movement between carcasses [16], it does not preclude synchronization of carcass dynamics through the movement of Diptera and Coleoptera adults from one carcass to another. The documentation of strong autocorrelation patterns in L. illustris and L. sericata under distances of a few kilometers indicates that the potential for this to occur is very real. The intention here is not to make forensic experiments any harder but rather to understand the consequences of the methodology used on the dynamics of decomposition and the organisms involved.
- Researchers should consider georeferencing experimental units at all scales (i.e., both the position of cadavers/carcasses in the field and the position of study sites for larger-scale projects). Multi-site studies cannot afford to ignore spatial and scale effects as these may be more influential than the variables under study.
- If researchers are not comfortable with spatial statistics, they can also choose other ways to incorporate spatial effects into their statistical models. For example, additive models lend themselves very well to the inclusion of geographic coordinates and autoregressive structures using, for example, tensor product smoothing. It is also possible to check the autocorrelation of residuals in most statistical procedures. Spatial analysis can be complex, but it is not necessary to know every facet of it to account for spatial interdependence.
This entry is adapted from the peer-reviewed paper 10.3390/insects13010011
References
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2001; p. 387.
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and diptera colonization. Forensic Sci. Int. 2001, 120, 18–27.
- Moreau, G.; Michaud, J.-P.; Schoenly, K.G. Experimental design, inferential statistics, and computer modeling. In Forensic Entomology: International Dimensions and Frontiers; Tomberlin, J.K., Benbow, M.E., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon on Thames, UK, 2015; pp. 205–230.
- Moreau, G. The Pitfalls in the Path of Probabilistic Inference in Forensic Entomology: A Review. Insects 2021, 12, 240.
- Cruickshank, I.; Wall, R. Aggregation and habitat use by Lucilia blowflies (Diptera: Calliphoridae) in pasture. Bull. Entomol. Res. 2002, 92, 153–158.
- Charabidze, D.; Gosselin, M.; Hedouin, V. Use of necrophagous insects as evidence of cadaver relocation: Myth or reality? PeerJ 2017, 5, e3506.
- Zabala, J.; Díaz, B.; Saloña-Bordas, M.I. Seasonal blowfly distribution and abundance in fragmented landscapes. Is it useful in forensic inference about where a corpse has been decaying? PLoS ONE 2014, 9, e99668.
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Commonly used intercarcass distances appear to be sufficient to ensure independence of carrion insect succession pattern. Ann. Entomol. Soc. Am. 2015, 109, 72–80.
- Nestel, D.; Carvalho, J.; Nemny-Lavy, E. The Spatial Dimension in the Ecology of Insect Pests and its Relevance to Pest Management. In Insect Pest Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 45–63.
- Wiens, J. A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385-397.
- Brundage, A.; Bros, S.; Honda, J.Y. Seasonal and habitat abundance and distribution of some forensically important blow flies (Diptera: Calliphoridae) in Central California. Forensic Sci. Int. 2011, 212, 115–120.
- Baz, A.; Cifrian, B.; Díaz-Äranda, L.M.; Martín-Vega, D. The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in Central Spain. Ann. Société Entomol. Fr. 2007, 43, 289–296.
- Tobler, W.R. A computer movie simulating urban growth in the detroit region. Econ. Geogr. 1970, 46, 234.
- Griffith, D. Towards a theory of spatial statistics. Geogr. Anal. 2010, 12, 325–339.
- Goguen, J.; Moreau, G. Exogenous and endogenous factors acting on the spatial distribution of a chrysomelid in extensively managed blueberry fields. Agric. For. Entomol. 2014, 17, 181–187.
- Schoenly, K.G.; Michaud, J.-P.; Moreau, G. Design and Analysis of Field Studies in Carrion Ecology. In Carrion Ecology, Evolution and Their Applications; Benbow, M.E., Tomberlin, J.K., Tarone, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 129–148.
- Kautz, M.; Schopf, R.; Ohser, J. The “sun-effect”: Microclimatic alterations predispose forest edges to bark beetle infestations. Eur. J. For. Res. 2013, 132, 453–465.
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613.
- Lewis, A.J.; Benbow, M.E. When entomological evidence crawls away: Phormia regina en masse larval dispersal. J. Med. Entomol. 2011, 48, 1112–1119.
- Fortin, M.J. Spatial Statistics in Landscape Ecology. In Landscape Ecological Analysis; Klopatek, J., Gardner, R., Eds.; Springer: New York, NY, USA, 1999; pp. 253–279.
- Amarasekare, P.; Nisbet, R.M. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am. Nat. 2001, 158, 572–584.
- Michaud, J.-P.; Majka, C.G.; Privé, J.-P.; Moreau, G. Natural and anthropogenic changes in the insect fauna associated with carcasses in the North American Maritime lowlands. Forensic Sci. Int. 2010, 202, 64–70.
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463.
- Boudreau, D.R.; Hammami, N.; Moreau, G. Environmental and evolutionary factors favouring the coexistence of sarcosaprophagous Calliphoridae species competing for animal necromass. Ecol. Entomol. 2021, 46, 1293–1300.
- Simpson, G.; Strongman, D.B. Carrion insects on pig carcasses at a rural and an urban site in Nova Scotia. Can. Soc. Forensic Sci. J. 2002, 35, 123–143.
- Kouki, J.; Hanski, I. Population aggregation facilitates coexistence of many competing carrion fly species. Oikos 1995, 72, 223.
- LeBlanc, K.; Boudreau, D.; Moreau, G. Small bait traps may not accurately reflect the composition of necrophagous Diptera associated to remains. Insects 2021, 12, 261.
- Michaud, J.-P.; Moreau, G. Predicting the visitation of carcasses by carrion-related insects under different rates of degree-day accumulation. Forensic Sci. Int. 2009, 185, 78–83.
- Marshall, S.A.; Whitworth, T.; Roscoe, L. Blow flies (Diptera; Calliphoridae) of eastern Canada with a key to Calliphoridae subfamilies and genera of eastern North America, and a key to the eastern Canadian species of Calliphorinae, Luciliinae and Chrysomyiinae. Can. J. Arthropod. Identif. 2011, 11.
- Johnson, M.D. Seasonal and microseral variations in the insect populations on carrion. Am. Midl. Nat. 1975, 93, 79.
- Woodcock, B.A.; Watt, A.D.; Leather, S.R. Aggregation, habitat quality and coexistence: A case study on carrion fly communities in slug cadavers. J. Anim. Ecol. 2002, 71, 131–140.
- Ricklefs, R.E. Community diversity: Relative roles of local and regional processes. Science 1987, 235, 167–171.
- Lutz, L.; Amendt, J.; Moreau, G. Carcass concealment alters assemblages and reproduction of forensically important beetles. Forensic Sci. Int. 2018, 291, 124–132.
- Anderson, G.S.; Van Laerhoven, S.L. Initial studies on insect succession on carrion in south western British Columbia. J. Forensic Sci. 1996, 41, 617–625.
- Bourel, B.; Luck, M.-B.; Hedouin, V.; Cailliez, J.-C.; Derout, D.; Gosset, D. Necrophilous insect succession on rabbit carrion in sand dune habitats in Northern France. J. Med. Entomol. 1999, 36, 420–425.
