Robotic surgery has gained much attention in liver resection for its potential to increase surgical dexterity in a minimally invasive scenario. In liver surgery, robotic systems help surgeons to localize tumors and improve surgical results with well-defined preoperative planning or increased intraoperative detection. Furthermore, they can balance the absence of tactile feedback and help recognize intrahepatic biliary or vascular structures during parenchymal transection. In addition, the robotic system presents the advantage of creating a hybrid interface in which pre- and intra-operative imaging tools could be exploited alone or together in order to guide surgical resection.
- robotic
- liver surgery
- augmented reality
1. Introduction
2. Augmented Reality
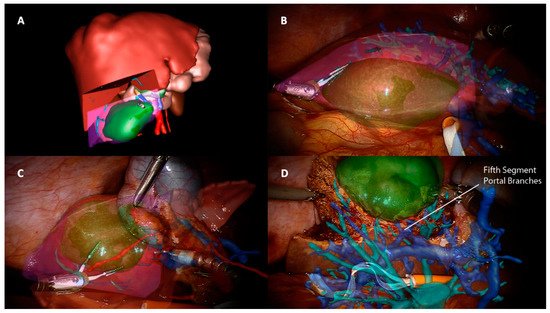
3. Image-Guided Robotic Liver Surgery
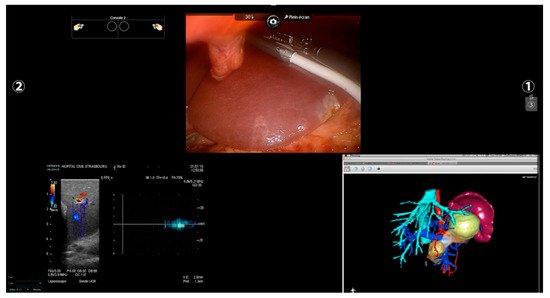
Other imaging strategies are more frequently used by surgeons during robotic liver resections in order to improve lesion detection and evaluate intraparenchymal biliary and vascular structures. Before exploring these modalities, a special mention goes to TilePro (Intuitive Surgical Inc., Sunnyvale, CA, USA). This is a multi-input display software integrated into the robotic platform, which shows more video sources simultaneously on the same screen (Figure 2). By simply connecting an external source to the Da Vinci console, surgeons and other operating room assistants can easily switch from the operating field to other input such as Intraoperative Ultrasound (IOUS) or preoperative cross-sectional imaging and 3D reconstructions.
3.1. Preoperative Imaging and 3D Rendering
3.2. Intra-Operative Robotic Ultrasound Application
3.3. Indocyanine Green Fluorescence
Another aspect to consider in ICG-guided resections is tumor clearance. Minimally invasive approaches—and robotic, in particular—lack a tactile feedback, and achieving a parenchymal free margin or performing an anatomical resection could be challenging. Furthermore, an IOUS exclusive evaluation could be insufficient because it is a user-dependent procedure and presents a heterogeneous detection rate according to tumor size and location and parenchymal stiffness [62,63,64]. In this context, fluorescence is a precious tool in robotic surgery, with some authors reporting an enlargement of the resection area after ICG application, both in benign ad malignant lesions, in order to achieve a R0 resection [57,65,66,67], and a significantly higher rate of margin-free specimens when comparing robotic hepatectomies with and without ICG [67]. As in open surgery, even in robotic surgery, some series described the detection of newer superficial lesions that the dye injection missed before [57]. This high sensitivity found is, however, limited to the liver surface because of the low penetration of the dye under 8 mm of depth, thus requiring the use of other imaging tools such as IOUS. Although no long-term results have been published, these findings have a significant impact in terms of oncological outcomes. ICG is a promising instrument of intraoperative navigation surgery, allowing rapid and easy identification of the resection plane without the inconveniences mentioned for other image-guided techniques. It can be used in combination with IOUS or AR as an additional aid rather than as a replacement [61] and with its features, it seems to fill some gaps found in robotic surgery, making tailored and oncological surgery less challenging.
4. Future Prospective
liver experience, mainly due to some technical limitations and to a relatively newborn and still debated approach [15]. AR, for example, is a time-consuming procedure, not only for the intraoperative installation, but also for preoperative planning and liver rendering [68]. In the context of an atypical or less demanding hepatic resection, which represent the first steps of a necessary learning curve, this time could appear exaggerated. Furthermore, AR in hepatic surgery has showed a delayed distribution compared to other surgical fields as neurosurgery, otolaryngology, orthopedics, and maxillofacial surgery [69,70,71]. This difference comes from anatomical obstacles, such as working with a deformable soft organ that is constantly moving during operation because of respiratory cycles as well as pneumoperitoneum creation [72]. Although some strategies have been described in this context [23,73,74], these features make the development of AR more complex, and new software are needed for shortening modeling creation and improving the accuracy of manual, semiautomatic, and automatic images overlapping.
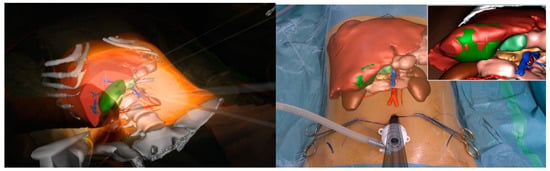
All the imaging techniques described must be seen, however, as a part of a puzzle rather than an independent solution towards a guided surgery; an example comes from registration accuracy in AR. IOUS and ICG have been proposed to improve overlapping quality through fluorescent markers and 3D ultrasounds used for intraoperative landmarks [75,76]. In this scenario, the robotic platform fits perfectly by creating a unique merged environment with the possibility of using and visualizing preoperative reconstruction and intraoperative images simultaneously within the operative field (Figure 4).
Another potential benefit of image-guided technology is minimally invasive training.
In laparoscopy, telementoring based on AR seems to speed up simple skills acquisition such as suturing [77] or even reduce the learning curve in more complex procedures such as cholecystectomy [78]. Similar applications in robotic training are lacking, with only a few experiences described [79]. Hepato-biliary surgery lacks standards of training and learning curves in robotic procedures [80], but recently, an expert panel of HPB surgeons agreed that a correct training path in hepatobiliary procedures needs different steps, starting from basic robotic skills before performing a liver resection [81]. In this context, AR could be a useful tool to support less-experienced surgeons performing simple procedures and lower their learning curve.
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cancers13246268
