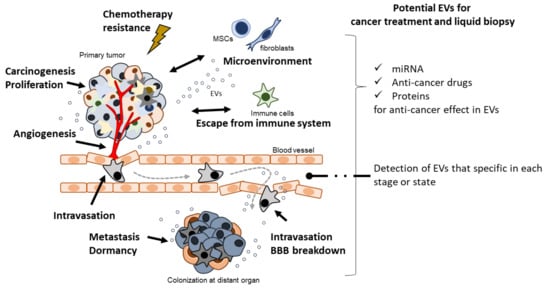
Cell–cell communication is an important mechanism in biological processes. Extracellular vesicles (EVs), also referred to as exosomes, microvesicles, and prostasomes, are microvesicles secreted from a variety of cells. Importantly, EVs contribute to cancer malignancy mechanisms such as carcinogenesis, proliferation, angiogenesis, metastasis, and escape from the immune system. As EVs are thought to be secreted into body fluids, they have the potential to serve as diagnostic markers for liquid biopsy. In addition, the characteristics of EVs make them suitable for use in drug delivery systems and novel cancer treatments.
1. Introduction
Extracellular vesicles (EVs), which also go by exosomes, microvesicles, and prostasomes, are secreted from a variety of cells [
1,
2] (
Table 1). EVs are nanometer-scale vesicles composed of a lipid bilayer and contain biologically functional molecules, such as microRNAs (miRNAs), mRNAs, and proteins [
3]. In recent years, EVs have been recognized as a cell–cell communication tool. The basic idea of cell–cell communication using EVs is that EVs secreted from donor cells are taken up by recipient cells in a paracrine or autocrine manner. EVs modify the condition of recipient cells by the biological molecules contained within them. The history of EVs dates back approximately 40 years, when Hans Lutz et al. reported the release of vesicles from old sheep erythrocytes [
4]. Ronquist reported a functional fraction in the supernatant of prostatic fluid in the same year [
5]. It has been reported that red blood cells secrete vesicles containing proteins and lipids during maturation [
6,
7,
8]. Some research groups have reported that the transferrin receptor is internalized in vesicles made by multivesicular bodies [
9,
10,
11]. However, in 1991, Johnstone et al. concluded that vesicles secreted from cells were “a garbage bin” for unnecessary membrane proteins [
12]. Importantly, the vesicles secreted from cells contain mRNA and miRNA, which can be transferred to other cells and be functional in them [
13].
Table 1. The features of extracellular vesicles.
| |
Extracellular Vesicles (EVs) |
| Terminology |
Exosomes |
Microvesicles |
Apoptotic Bodies |
| Origin |
Multivesicle body |
Plasma membrane |
Plasma membrane |
| Size |
50–150 nm |
100–1000 nm |
100–5000 nm |
| Marker proteins |
CD9, CD63, Tsg101 etc. |
Integrins, Selectins, CD40 etc. |
Annexin V, thrombospondin, C3b etc. |
| References |
[1,3] |
[1,23] |
[1,24] |
In the past two decades, EVs have been shown to play a crucial role in cancer biology. Accumulating evidence indicates the importance of cell–cell communication through EVs in cancer malignancy mechanisms, such as cancer cell proliferation [16], immune modulation [17], angiogenesis [18], metastasis [19], and pro-metastasis niche formation [20] (Figure 1). Importantly, in the early history of EV research, EVs derived from dendritic cells (DCs) pulsed with tumor peptides were proposed as a cell-free vaccine method [21]. Furthermore, a potential cancer therapeutic strategy based on the suppression of cancer metastasis via the removal of EVs that contribute to cancer malignancy has been reported [22].
Figure 1. EVs contribute to cancer malignancy and they have an emerging role of therapeutic potential in cancer malignancy.
2. Carcinogenesis
Cancer cells emerge from cells damaged by various factors, such as inflammation, chemical stress, radiation, oxidative stress, and aging [
117]. These stresses, especially aging, affect the formation of malignant tumors through the accumulation of genetic and epigenetic changes in genes. It is likely that there is a relationship between these causes of carcinogenesis. Chronic inflammation, such as “inflammaging,” is a risk factor for carcinogenesis, and is caused by cytokines and chemokines [
118]. It is predicted that elderly people probably have chronic inflammation without infection caused by senescent cells. The senescence-associated secretory phenotype is a feature of senescent cells that leads to chronic inflammation in elderly people, with factors such as interleukin (IL) -6, and IL-8 secreted by senescent cells [
119]. EVs are also secreted from senescent cells, and they may exert detrimental effects [
120,
121]. Accumulating evidence suggests that EVs contribute to carcinogenesis or precancerous conditions, such as inflammation [
26,
122,
123,
124], fibrosis [
25,
125,
126], double-strand breaks in DNA [
27,
126], and endoplasmic reticulum (ER) stress [
127].
Bladder cancer cell-derived EVs induce neoplastic transformation of nonmalignant cells through the induction of the unfolded protein response in the ER [
127]. EVs have been suggested to affect tumor recurrence and the potential for carcinogenesis. The Epstein–Barr virus M81-infected B cells release EVs that contain non-coding Epstein–Barr virus-encoded RNA, which are then taken up by B cells [
26], which results in chronic inflammation. Inflammation and carcinogenesis caused by viral infection may be linked. EVs derived from macrophages have been reported to upregulate TBC1 domain family member 3 by downregulating stanniocalcin-1-mediated inflammation [
122].
3. Proliferation
“Enabling replicative immortality” is a hallmark of cancer [
129]. HeLa cells were established in 1953 and contribute greatly to cancer research because of their proliferation on a dish [
130]. It is well known that activation of telomerase is one of the causes of proliferation, as it is not subject to the Hayflick limit. Uncontrolled proliferation is a fundamental feature of cancer that leads to gene mutations, metabolic changes, and epigenetic alterations possibly resulting in malignancy. Cancer cell-derived EVs contribute to cell proliferation and growth [
131,
132,
133,
134,
135].
EVs from mesenchymal stem cells (MSCs) play a dual role in cancer biology. They exhibit potential as anti-cancer treatments but also contribute to cancer malignancy [
131,
136]. Importantly, MSCs are educated by cancer-derived EVs to contribute to cancer malignancy. It has been reported that EVs derived from cholangiocarcinoma-educated bone marrow MSCs enhance the secretion of C-X-C motif chemokine ligand (CXCL)-1, C-C motif chemokine ligand 2 (CCL2), and IL-6, which affect cancer proliferation [
131]. MiR-410 containing EVs derived from human umbilical cord MSCs decreases phosphatase and tensin homolog (PTEN) expression in lung adenocarcinoma. These results suggests that the uptake of EVs by lung adenocarcinoma increases proliferation and decreases apoptosis. Cancer-associated fibroblasts (CAFs) also play a crucial role in cancer proliferation [
29].
4. Angiogenesis and Intravasation
Angiogenesis and lymphangiogenesis are important for the survival and progression of cancer cells, which are activated by signals from cancer cells that are growing [
138]. These are important steps for the supply of oxygen, nutrients, and metabolism in cancer cells [
139]. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor, angiogenin, and TGF-α play an important role in angiogenesis [
139]. Cancer-derived EVs also play an important role in angiogenesis [
134,
140,
141,
142,
143,
144,
145,
146] and lymph-angiogenesis [
147]. EVs derived from colorectal cancer cells activate early growth response protein-1 in endothelial cells, causing the migration of endothelial cells [
34]. Cancer-derived EVs stimulate MSCs to form vessel-like formations [
148]. It has been reported that miRNAs in EVs also plays an important role in angiogenesis [
45]. Rac1-, PAK2- [
44], VEGF- [
46], and angiopoietin-2-containing [
48] EVs are related to angiogenesis. These results suggest that cancer-derived EVs promote angiogenesis. Lymph nodes are a route of cancer metastasis [
149]. It is reported that laminin γ2-containing EVs promote lymphangiogenesis [
50]; however, mechanisms underlying lymphatic intravasation remain unclear.
5. Metastasis
Cancer cells can metastasize to any part of the body; however, sites such as bone, the liver, and the lungs are the most common. Brain metastasis is a critical cause of death. Uncontrolled cancer metastasis is a major cause of cancer-related deaths. Cancer metastasis involves multiple steps, such as epithelial–mesenchymal transition (EMT), intravasation, extravasation, and proliferation at the metastatic organ. The seed-and-soil theory is well accepted as a mechanism of metastasis [
150]. Using the metastatic efficiency index, Weiss suggested that 65% of metastasis seems to be caused by the amount of organ blood flow [
150,
151]. In contrast, there are common sites of cancer metastasis and sites that are specific to the cancer type. In such complicated mechanisms of metastasis, EVs contribute to EMT [
152,
153,
154], migration [
63,
155,
156,
157], metastasis niche formation [
20,
158,
159], metastasis promotion [
158], and the tumor microenvironment [
160].
6. Escape from Immune System
The immune system consists of macrophages, B cells, T cells, and DCs. It protects the body from invaders such as bacteria, viruses, and toxins; contributes to the recovery of the body; and removes cancer cells. However, cancer cells can escape the immune system by modifying them through EVs. Programmed death ligand-1 (PD-L1) is a receptor that suppresses or stops T-cell reactions by binding to programmed cell death-1 (PD-1). EVs derived from glioblastoma promote immune evasion through PD-1 binding to PD-L1 on EVs [
69,
70]. Ovarian cancer cell-derived EVs have been reported to inhibit T-cell receptor-dependent activation in T-cells [
177]. EVs derived from tumor-associated macrophages have immunosuppressive effects; conversely, these cells have the potential to activate anti-tumor immunity [
178]. MSC-derived EVs containing miR-222 contribute to immune escape in colorectal cancer by downregulating the AKT pathway [
65]. These reports suggest that EVs derived from cancer cells and cells in the microenvironment suppress the immune system.
7. Chemotherapeutic Stress
Bone marrow MSC-derived EVs increase the viability of multiple myeloma cells and drug resistance [
181]. Non-small-cell lung cancer cell-derived EVs increase gefitinib-induced apoptosis [
182]. In EVs, lncRNAs contribute to drug resistance [
33,
75,
76,
77,
78,
79], with lncRNA H19 in EVs increasing gefitinib resistance in non-small-cell lung cancer cells [
73]. EVs derived from MSCs increase drug resistance [
157]. MSC-EVs are collected under stress using a non-serum culture medium. EVs derived from under-stressed MSCs decrease doxorubicin-induced apoptosis in osteosarcoma cells, and those derived from melanoma cells containing anaplastic lymphoma kinase could transfer drug resistance to other melanoma cells [
71]. EVs derived from tumor-associated macrophages, which are components of the cancer microenvironment, increase resistance to the anticancer drug gemcitabine [
74]. MiR-30b-3p in EVs derived from hypoxic glioma cells contributes to drug resistance by decreasing the expression of ras homolog family member B [
72].
8. Potential of EVs for Liquid Biopsy
EVs secreted into the extracellular environment may be related to mechanisms of cancer malignancy. Many reports have suggested that EVs contain specific molecules that contribute to cancer malignancy or related cancer types. Therefore, it is possible that EVs can be used for diagnosis. A liquid biopsy is a test that uses body fluids such as blood, bone marrow, saliva, urine, and tears. A minimally invasive method is required in liquid biopsy as much as possible to avoid pain. Bone marrow biopsy is an invasive method, as is blood biopsy. On the other hand, saliva, urine, and tears are non-invasive methods of biopsy. Liquid biopsy tests can identify early stage, progression, and metastasis of cancer by detecting specific molecules. The idea of a liquid biopsy using EVs is to detect cancer-specific EV molecules such as miRNAs, DNAs, and proteins for the diagnosis of cancer [
184].
9. Potential of EVs for Cancer Treatment
The idea of cancer treatment using EVs has three aspects—(1) inhibition of EV production, (2) disruption of EV uptake, and (3) elimination of EVs [
215]. In addition, there is a strategy for the application of EVs that carry a druggable molecule for cancer treatment [
216]. CD63 and CD9 are used as markers for targeting EVs in the circulation. EV elimination from the circulation using anti-CD63 and CD9 antibodies can reduce cancer metastasis [
22]. The CD9 Fab fragment also inhibits EV internalization [
100]. These reports suggest that targeting proteins on EVs can be used for the neutralization and elimination of EVs from the blood. It has also been suggested that potential therapeutics exert anti-cancer effects by blocking EVs containing TGF-β [
217]; however, the therapeutics strategy of “inhibition of EV production” is still unclear.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13246303