Transition metal-catalyzed carbonylation reactions have emerged as one of the most relevant synthetic approaches for the preparation of carbonyl-containing molecules. The most commonly used protocol for the insertion of a carbonyl moiety is the use of carbon monoxide (CO) but, due to its toxic and explosive nature, this process is not suitable at an industrial scale. The chemistry of CO surrogates has received large attention as a way to use less expensive and more environmentally friendly methods. Among the various CO surrogates, N,N-dimethylformamide (DMF) has been paid greater attention due to its low cost and easy availability.
- carbonylation
- catalytic processes
- CO surrogates
- DMF
- heterocycles
- synthetic methods
1. Introduction
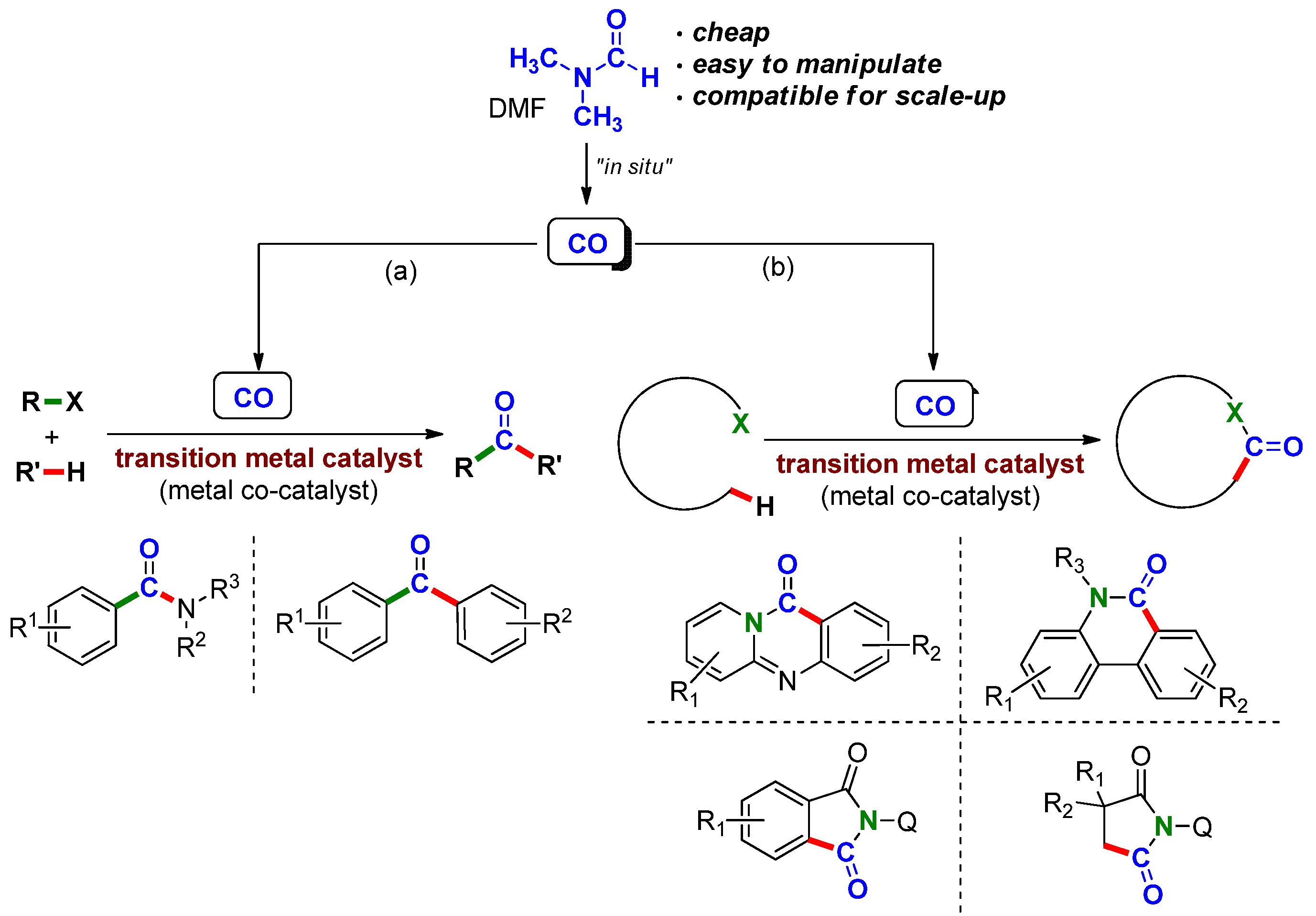
2. Synthesis of Heterocycles by Transition Metal-Catalyzed Carbonylation Reactions Involving DMF as a CO Surrogate
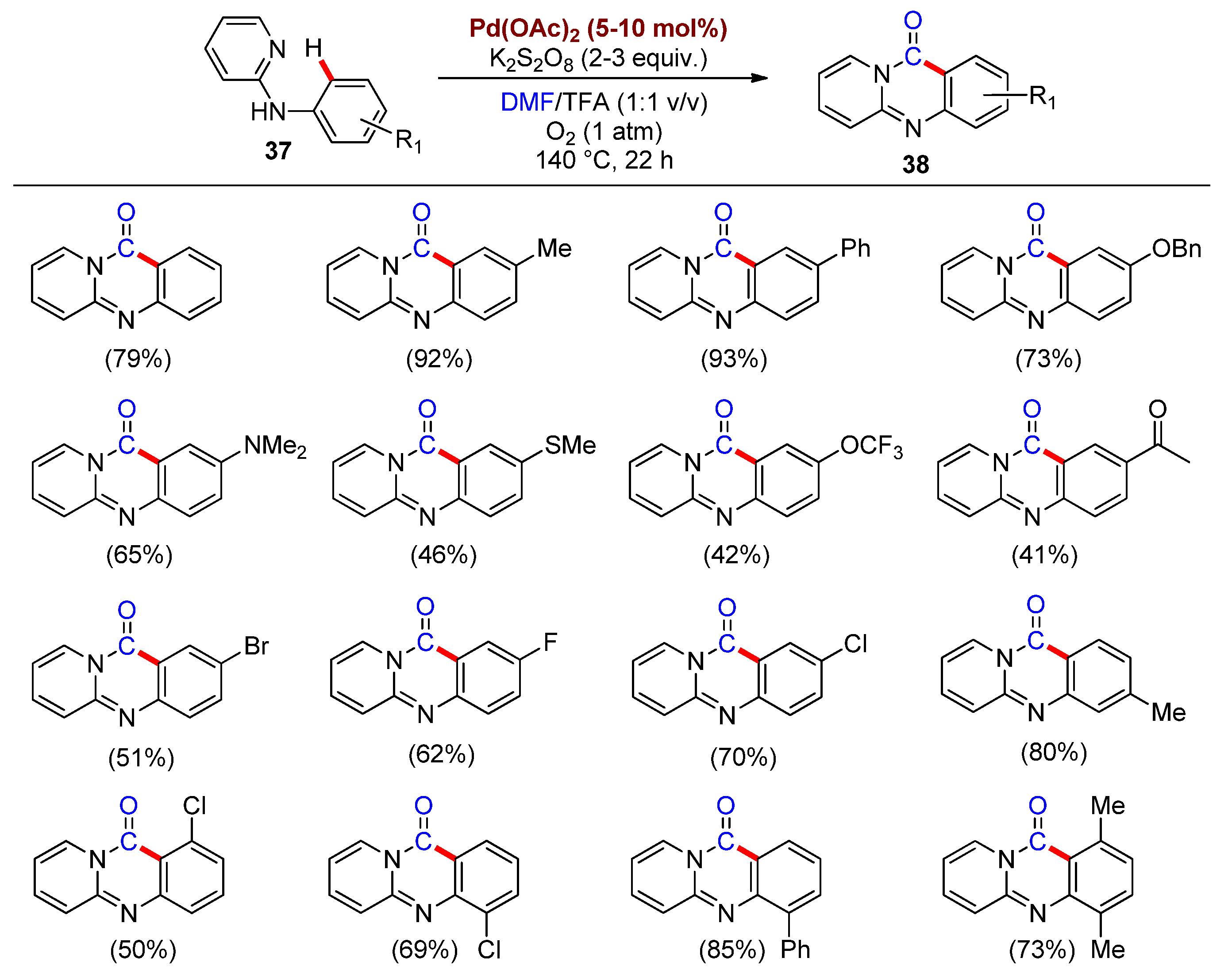
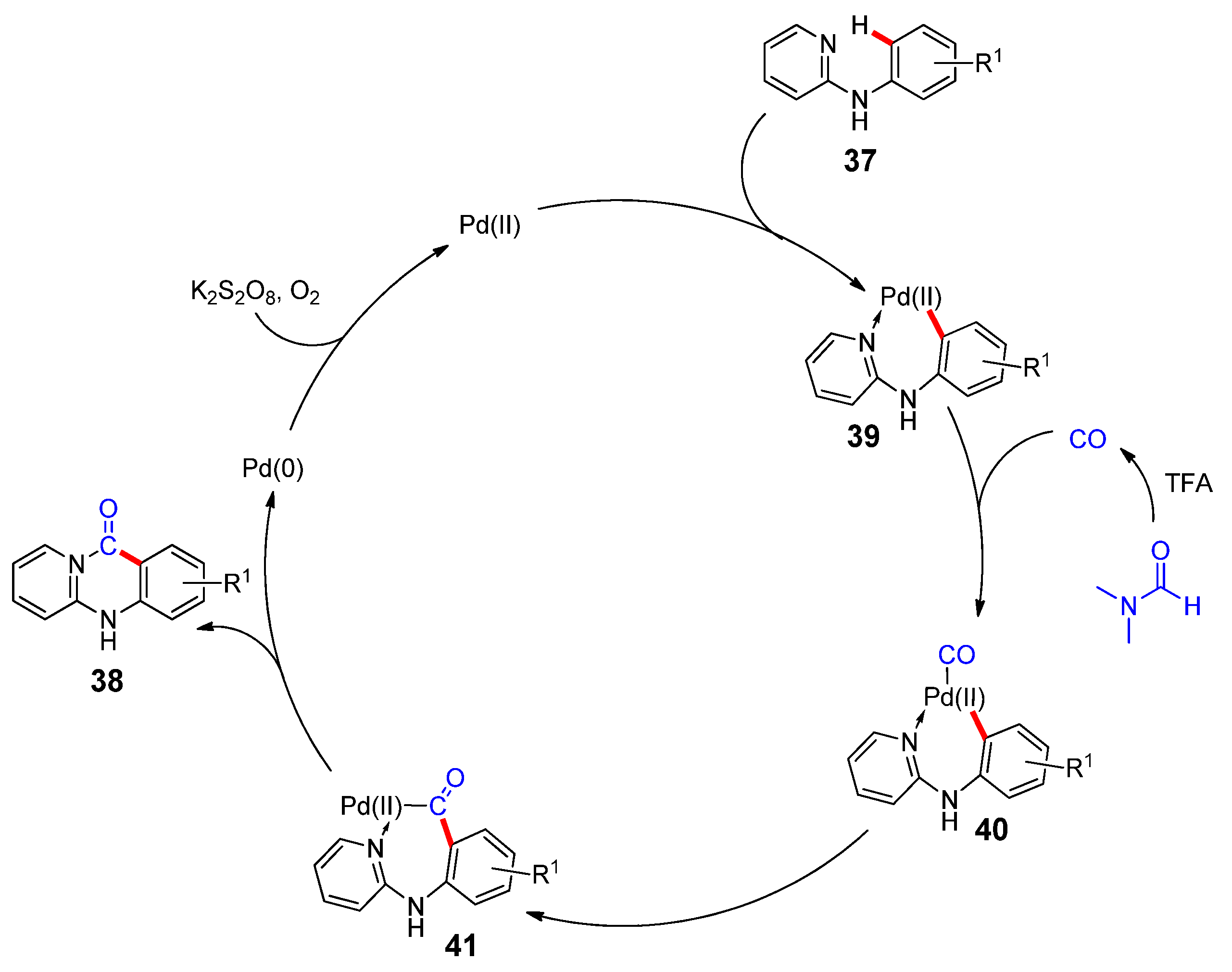
2.1. Synthesis of Heterocycles by Palladium-Catalyzed Carbonylation Involving DMF as a CO Surrogate
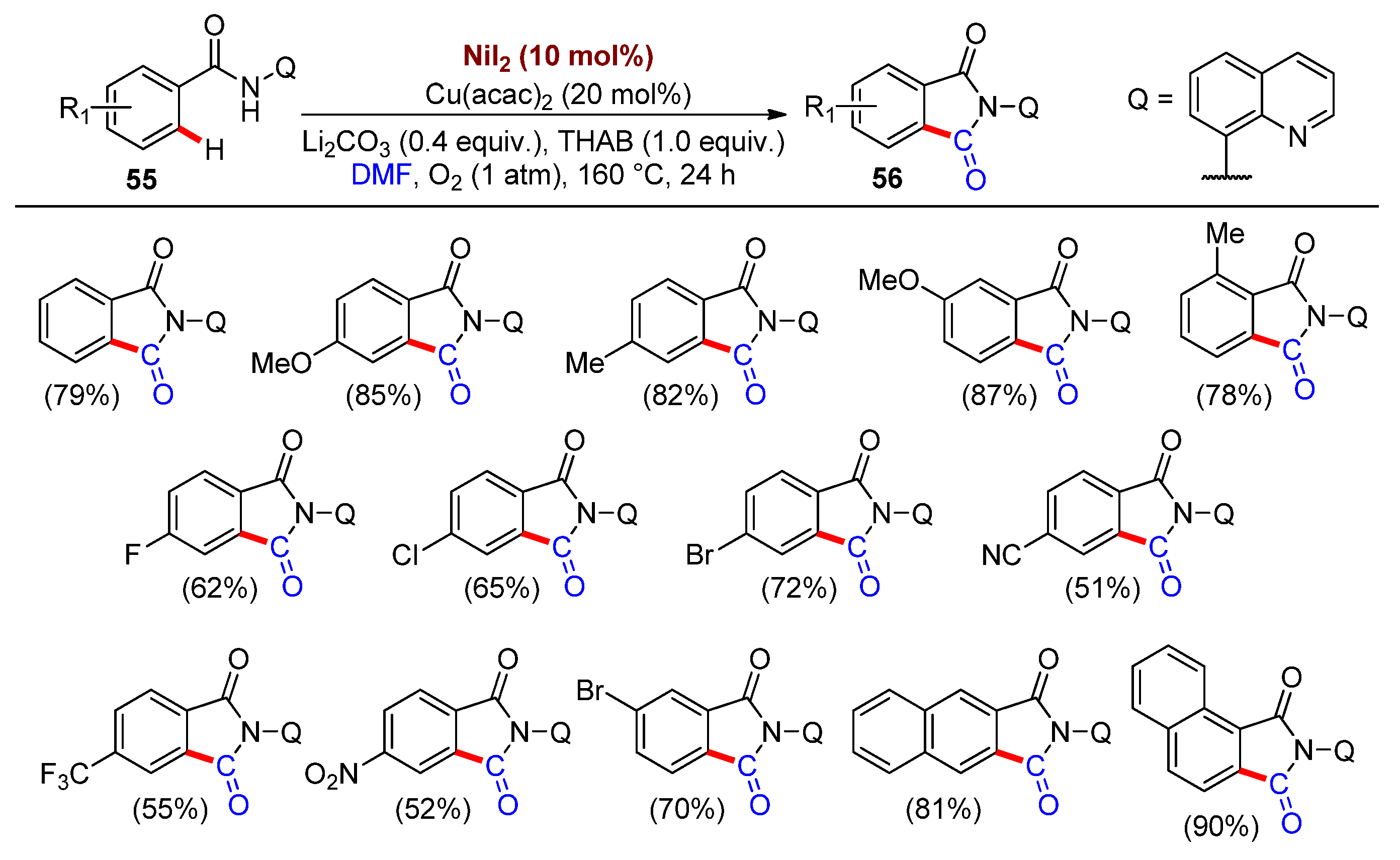
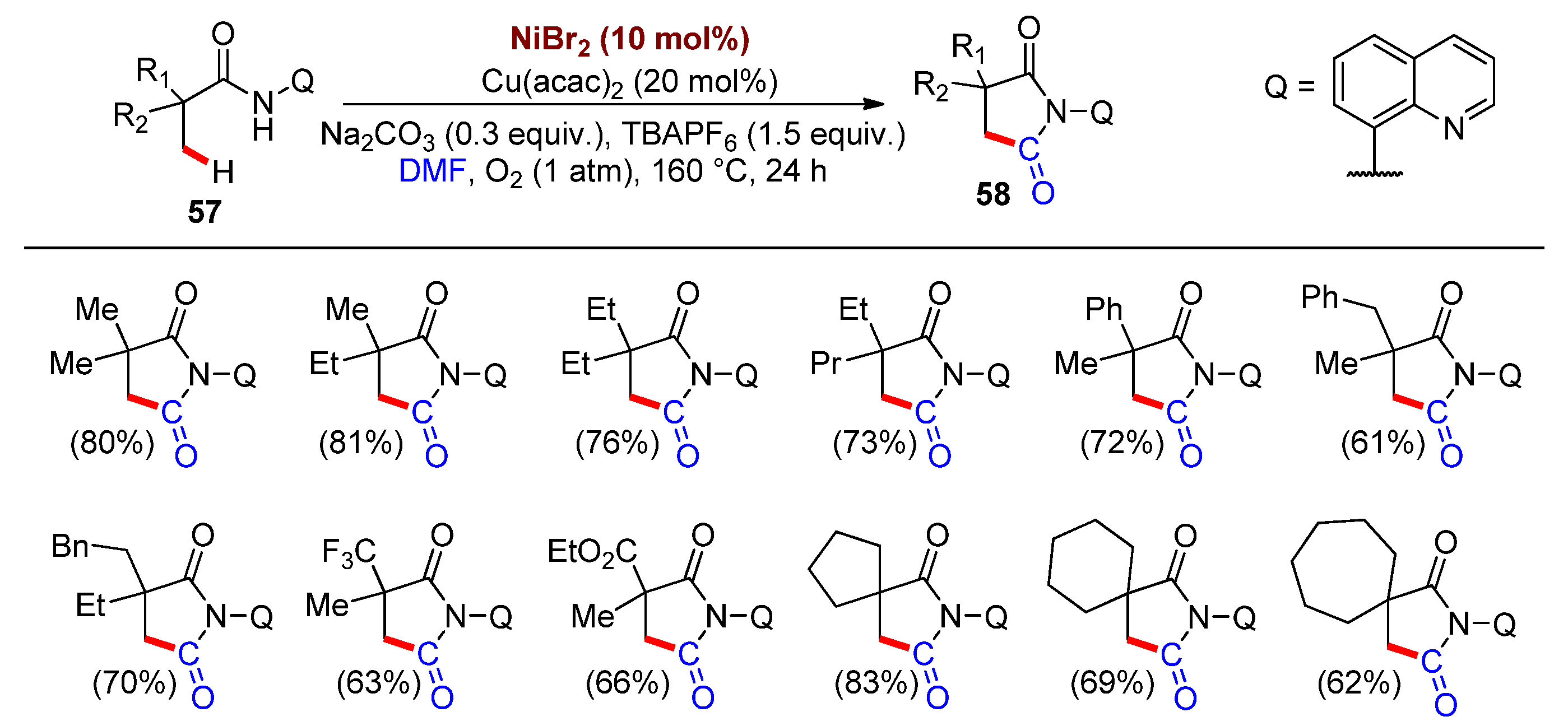
2.2. Synthesis of Heterocycles by Nickel-Catalyzed Carbonylation Involving DMF as a CO Surrogate
3. Critical Aspects and Perspectives in the Synthesis of Heterocycles by Transition Metal-Catalyzed Carbonylation Reactions Involving DMF as a CO Surrogate
This entry is adapted from the peer-reviewed paper 10.3390/catal11121531
References
- Semchyshyn, H.M. Reactive Carbonyl Species In Vivo: Generation and Dual Biological Effects. Sci. World J. 2014, 2014, 417842.
- Li, M.; Zajaczkowski, W.; Velpula, G.; Jänsch, D.; Graf, R.; Marszalek, T.; Parekh, S.H.; Zagranyarski, Y.; Mali, K.; Wagner, M.; et al. Transformation from helical to layered supramolecular organization of asymmetric perylene diimides via multiple intermolecular hydrogen bonding. Chem. Sci. 2020, 11, 4960–4968.
- Hassan Omar, O.; Falcone, M.; Operamolla, A.; Albano, G. Impact of chirality on the aggregation modes of L-phenylalanine- and D-glucose-decorated phenylene–thiophene oligomers. New J. Chem. 2021, 45, 12016–12023.
- Schoenberg, A.; Bartoletti, I.; Heck, R.F. Palladium-catalyzed carboalkoxylation of aryl, benzyl, and vinylic halides. J. Org. Chem. 1974, 39, 3318–3326.
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar–X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009.
- Peng, J.-B.; Qi, X.; Wu, X.-F. Recent Achievements in Carbonylation Reactions: A Personal Account. Synlett 2017, 28, 175–194.
- Dekleva, T.W.; Forster, D. The rhodium-catalyzed carbonylation of linear primary alcohols. J. Am. Chem. Soc. 1985, 107, 3565–3567.
- Haynes, A.; Maitlis, P.M.; Morris, G.E.; Sunley, G.J.; Adams, H.; Badger, P.W.; Bowers, C.M.; Cook, D.B.; Elliott, P.I.P.; Ghaffar, T.; et al. Promotion of Iridium-Catalyzed Methanol Carbonylation: Mechanistic Studies of the Cativa Process. J. Am. Chem. Soc. 2004, 126, 2847–2861.
- Morimoto, T.; Kakiuchi, K. Evolution of Carbonylation Catalysis: No Need for Carbon Monoxide. Angew. Chem. Int. Ed. 2004, 43, 5580–5588.
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of Alkenes with CO Surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310–6320.
- Gautam, P.; Bhanage, B.M. Recent advances in the transition metal catalyzed carbonylation of alkynes, arenes and aryl halides using CO surrogates. Catal. Sci. Technol. 2015, 5, 4663–4702.
- Chen, J.; Natte, K.; Spannenberg, A.; Neumann, H.; Beller, M.; Wu, X.-F. Palladium-Catalyzed Carbonylative Annulation of N-Aryl-Pyridine-2-Amines with Internal Alkynes by C–H Activation: Facile Synthesis of 2-Quinolinones. Chem. Eur. J. 2014, 20, 14189–14193.
- Cao, J.; Zheng, Z.-J.; Xu, Z.; Xu, L.-W. Transition-metal-catalyzed transfer carbonylation with HCOOH or HCHO as non-gaseous C1 source. Coord. Chem. Rev. 2017, 336, 43–53.
- Li, H.; Neumann, H.; Beller, M.; Wu, X.-F. Aryl Formate as Bifunctional Reagent: Applications in Palladium-Catalyzed Carbonylative Coupling Reactions Using In Situ Generated CO. Angew. Chem. Int. Ed. 2014, 53, 3183–3186.
- Natte, K.; Dumrath, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylations of Aryl Bromides using Paraformaldehyde: Synthesis of Aldehydes and Esters. Angew. Chem. Int. Ed. 2014, 53, 10090–10094.
- Shil, A.K.; Kumar, S.; Reddy, C.B.; Dadhwal, S.; Thakur, V.; Das, P. Supported Palladium Nanoparticle-Catalyzed Carboxylation of Aryl Halides, Alkenylsilanes, and Organoboronic Acids Employing Oxalic Acid as the C1 Source. Org. Lett. 2015, 17, 5352–5355.
- Lescot, C.; Nielsen, D.U.; Makarov, I.S.; Lindhardt, A.T.; Daasbjerg, K.; Skrydstrup, T. Efficient Fluoride-Catalyzed Conversion of CO2 to CO at Room Temperature. J. Am. Chem. Soc. 2014, 136, 6142–6147.
- Mondal, K.; Halder, P.; Gopalan, G.; Sasikumar, P.; Radhakrishnan, K.V.; Das, P. Chloroform as a CO surrogate: Applications and recent developments. Org. Biomol. Chem. 2019, 17, 5212–5222.
- Albano, G.; Aronica, L.A. Potentiality and Synthesis of O- and N-Heterocycles: Pd-Catalyzed Cyclocarbonylative Sonogashira Coupling as a Valuable Route to Phthalans, Isochromans, and Isoindolines. Eur. J. Org. Chem. 2017, 2017, 7204–7221.
- Aronica, L.A.; Albano, G.; Giannotti, L.; Meucci, E. Synthesis of N-Heteroaromatic Compounds through Cyclocarbonylative Sonogashira Reactions. Eur. J. Org. Chem. 2017, 2017, 955–963.
- Albano, G.; Giuntini, S.; Aronica, L.A. Synthesis of 3-Alkylideneisoindolin-1-ones via Sonogashira Cyclocarbonylative Reactions of 2-Ethynylbenzamides. J. Org. Chem. 2020, 85, 10022–10034.
- Albano, G.; Evangelisti, C.; Aronica, L.A. Palladium Nanoparticles Supported on Smopex-234® as Valuable Catalysts for the Synthesis of Heterocycles. Catalysts 2021, 11, 706.
- Albano, G.; Morelli, M.; Aronica, L.A. Synthesis of Functionalised 3-Isochromanones by Silylcarbocyclisation/Desilylation Reactions. Eur. J. Org. Chem. 2017, 2017, 3473–3480.
- Albano, G.; Morelli, M.; Lissia, M.; Aronica, L.A. Synthesis of Functionalised Indoline and Isoquinoline Derivatives through a Silylcarbocyclisation/Desilylation Sequence. ChemistrySelect 2019, 4, 2505–2511.
- Albano, G.; Aronica, L.A. From Alkynes to Heterocycles through Metal-Promoted Silylformylation and Silylcarbocyclization Reactions. Catalysts 2020, 10, 1012.
- Chen, Z.; Wang, L.-C.; Wu, X.-F. Carbonylative synthesis of heterocycles involving diverse CO surrogates. Chem. Commun. 2020, 56, 6016–6030.
- Ding, S.; Jiao, N. N,N-Dimethylformamide: A Multipurpose Building Block. Angew. Chem. Int. Ed. 2012, 51, 9226–9237.
- Chen, J.; Feng, J.-B.; Natte, K.; Wu, X.-F. Palladium-Catalyzed Carbonylative Cyclization of Arenes by C-H Bond Activation with DMF as the Carbonyl Source. Chem. Eur. J. 2015, 21, 16370–16373.
- Wu, X.; Zhao, Y.; Ge, H. Direct Aerobic Carbonylation of C(sp2)–H and C(sp3)–H Bonds through Ni/Cu Synergistic Catalysis with DMF as the Carbonyl Source. J. Am. Chem. Soc. 2015, 137, 4924–4927.
- Chen, J.; Natte, K.; Wu, X.-F. Pd/C-catalyzed carbonylative C–H activation with DMF as the CO source. Tetrahedron Lett. 2015, 56, 6413–6416.
- Nageswar Rao, D.; Rasheed, S.; Das, P. Palladium/Silver Synergistic Catalysis in Direct Aerobic Carbonylation of C(sp2)−H Bonds Using DMF as a Carbon Source: Synthesis of Pyrido-Fused Quinazolinones and Phenanthridinones. Org. Lett. 2016, 18, 3142–3145.
