In diagnosing SARS-CoV-2 infection, the most widely used test is the molecular testing. Real-time reverse transcription polymerase chain reaction (RT-PCR) is the most well-known and extensively used molecular analysis. The test relies on nucleic acid amplification and detects unique sequences of SARS-CoV-2. The other type of test, the antigen tests, can detect the presence of SARS-CoV-2 without amplifying viral components, but these tests are less sensitive than the molecular ones. Commonly, any negative antigen test is confirmed with a molecular test so that the patient can be declared negative for COVID-19. Both molecular and antigen tests would detect patients in the acute phase of infection.
1. Introduction
Laboratory diagnosis in COVID-19 is influential in combating the spreading of SARS-CoV-2 infection. Moreover, laboratory tests dictate the clinical decisions regarding the infected patient. These tests comprise the ones that detect the viral genome and testes that detect the viral proteome. Upon molecular and antigen tests, patients were classified as positive or negative for the presence of SARS-CoV-2. Nevertheless, all tests have two seminal characteristics/parameters, namely, percent positive agreement (PPA), describing the actual sensitivity of the test, and percent negative agreement (PNA), describing the specificity of the test [3].
In diagnosing SARS-CoV-2 infection, the most widely used test is the molecular testing. Real-time reverse transcription polymerase chain reaction (RT-PCR) is the most well-known and extensively used molecular analysis. The test relies on nucleic acid amplification and detects unique sequences of SARS-CoV-2 [4]. The other type of test, the antigen tests, can detect the presence of SARS-CoV-2 without amplifying viral components, but these tests are less sensitive than the molecular ones. Commonly, any negative antigen test is confirmed with a molecular test so that the patient can be declared negative for COVID-19. Both molecular and antigen tests would detect patients in the acute phase of infection [5,6].
2. Technologies to Assess Specific Antigens
2.1. Quantitative Real-Time Reverse Transcriptase-PCR
RT-PCR is a technology used on a large scale for diagnosing different viral infections, such as Ebola and Zika infection. Therefore, when this new coronavirus infection hit the world, the already used technology expanded for this virus.
Viral RNA is detected using RT-PCR, and the test reports the abundance of viral genetic material, with results being reported as “qualitative”. In this test, the abundance detected above an established threshold gives the already famous “positive” results. Establishing the appropriate threshold is probably the most argued issue in this pandemic. The main purpose of the imposed threshold is not to miss false negative results while minimizing false positives [
8].
In a nutshell, the technology consists of two clear stages: viral RNA is reverse transcribed into DNA, that is amplified due to polymerase chain reaction (PCR) [
9]. FDA and the Centre for Disease Control and Prevention (CDC) recommended, for this test, several regions to be detected: viral nucleocapsid N1, N2, and human RNase P gene [
9]. The World Health Organization (WHO) has recommended detecting CoV-2 RNA-dependent RNA polymerase (RdRP) and envelope (E) genes [
10].
An infectious virus particle has intact nucleic acid covered by a capsid. Starting from this assertion, there are several points that need more attention. Hence, RT-PCR detects viral RNA, but this genetic material is not mandatorily appended to a replicating virus [
11,
12]. There are studies that show the relation between a cultivable virus and the viral RNA that is shed. Moreover, recovering patients, although not infectious anymore, are still shedding viral RNA [
13]. When sampling a nasopharyngeal swab, proteins and debris are eliminated and the extricated RNA is tested, but this RNA contains both individual and viral RNA. Hence the entire extracted RNA is reverse transcribed into DNA and amplified by PCR. For major viral RNA loads, the reverse-transcribed DNA will have mainly viral genetic information, but samples with borderline viral load will not have such clear results [
13,
14].
RT–PCR is an end-point technology; it will not give information on past infection, information needed for registering epidemiological events. The only test that can directly indicate if the infectious viral particles are still present is the viral cultivation in particular cells, such as African green monkey kidney Vero C1008 clone E6 cells [
15].
Viral load in an individual is associated with the severity of the infection, but this issue has also things that should be clarified [
16]. Studies regarding the relation between viral load and clinical evolution are still very few, the retrospective nature of the investigation is limited, and sample sizes and selection bias are still not relevant. Another issue that is raised in this field is the PCR type used to measure the viral infection. Sample type, patient’s age and gender, comorbidities, and probably many more factors influence the viral load, parameters that are still to be established [
17] along with the viability and infectiousness of the virus [
18]. All these parameters should be related to the results that RT-PCR test would provide.
2.2. Nonconventional Tests—Droplet-Digital PCR
As current diagnostic tests are based on the RT-qPCR method, several limitations in terms of sensitivity and quantification have emerged. To improve its performance, new, improved tests are developed. Thus, in a study, published in March 2021, qPCR and droplet digital PCR (ddPCR) were tested for their capability to detect low amounts of viral RNA. ddPCR is a highly sensitive technology that uses a water–oil emulsion droplet system so that nucleic acid samples are partitioned in 20,000 nanoliter-sized droplets serving as independent test tubes [
19]. Each sample would have thousands of individual partitions, with or without template DNA [
20]. A PCR reaction develops in each tube and is examined for amplified target DNA by fluorescence [
21]. The limit of detection of ddPCR is about 0.005%, much lower when compared to that of RT-PCR (1%), pyrosequencing (5%), melting curve analysis (10%), and Sanger sequencing (20%) [
22]. This technology can overpass RT-PCR because it brings absolute quantification of DNA copies without using external calibration curves. The test bypasses known PCR inhibitors and hence provides higher accuracy, reproducibility, and increased sensitivity, especially for low concentrations of the searched molecules or degraded samples [
20,
23].
When directly comparing ddPCR with RT-PCR, the cycle threshold (CT) of the viral RNA identified by RT-PCR significantly varied related to the sequences of the primer and probe sets, while the copy number of the viral RNA depicted by ddPCR was effectively quantified with in vitro transcript RNA, cultured viral RNA, and RNA from clinical samples. Authors conclude that ddPCR could be used as an extremely sensitive and compatible diagnostic method for viral RNA detection [
24]. Another group has developed a multiplex ddPCR for sensitive quantification of specific RNA with respect to human-derived RNA in screening and monitoring COVID-19 patients. This multiplex ddPCR detects, simultaneously, SARS-CoV-2 E, RdRp, and N viral RNA, and human Rpp30 DNA and GUSB mRNA (internal nucleic acid extraction and control). De Kock et al. proved that RT-ddPCR assay sensitivity was not affected by the total nucleic acids background. This is not the case for classical standard RT-PCR because total nucleic acids affect sensitivity [
25]. Another multiplex ddPCR analysis was tested by Deiana et al. Comparing swabs with or without RNA extraction, the group has shown that the direct approach generated equal RNA copies in comparison to the extracted ones. Therefore, using ddPCR direct quantitation of virus SARS-CoV-2 in nasopharyngeal swab yielded an efficient quantitation [
26]. Molecular analysis performed in patients’ plasma using ddPCR in comparison to classical PCR has shown also encouraging results. In plasma harvested from COVID-19 patients diagnosed in mild, moderate, and critical disease, virus was detected in 91% of patients when using ddPCR and in 87% when using RT-PCR. Both methods could detect RNAemia with ICU patients having the highest prevalence [
27].
However, despite its high sensitivity, high specificity, and its potential clinical utility, the ddPCR approach implies high financial resources and highly trained personnel, making this powerful method still unaffordable for low-income countries.
2.3. Antigen Detection Tests
Another SARS-Cov-2 test method for diagnosing an active case is the antigen test, useful in early stages of the infection. The tests can detect viral antigen in the nasal, oral, and respiratory tract, sites in which the virus is actively shed, and hence has the highest infectivity.
These tests can detect viral presence up to 2 days before the onset of symptoms and are easy to perform. As specific antibodies are detectable at the earliest within the first week from the symptom onset, antigen tests can detect early infection. The test results depend on the duration of viral shedding and on several clinical parameters, such as disease severity, duration of the illness, and patient’s immune response. Viral shedding becomes undetectable around one month after symptoms onset or much earlier when the symptoms disappear.
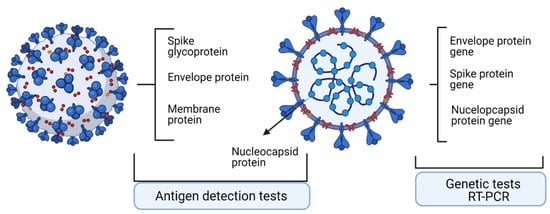
Antigen tests can identify nucleocapsid (N) or spike (S) proteins [
9]. In antigen testing, N is a good target because it is a conserved and abundant antigen. The antigen tests can have enzyme-linked immunosorbent assay (ELISA) format or a lateral flow rapid-test format. The ELISA format relies on the existence of a pair of specific antibodies that would recognize the target antigen, the tests having high sensitivity and specificity.
The ELISA format gives accurate results but needs above-average equipment and staff to handle the methodology, these issues being somewhat limiting in less-developed laboratories.
The lateral flow format or antigen rapid test can be used in a general screening of a population and can be handled even by nonmedical laboratories. It resembles HIV rapid test using serum, plasma, or fingertip blood. The rapid test gives visually interpretable results in around 20 min, being a point-of-care (POC) setting.
Supplementary tests that aid the clinical management of the infected patients are currently used in several units; these comprise coagulation tests, indicators of cytokine storm (e.g., interleukin-6), ferritin, granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein–1α (MIP-1α), and tumor necrosis factor–α (TNF-α) [
28].
A summary table of genome and proteome testing in viral infection [
3] is presented in
Table 1, and a schematic outline of the tests is shown in
Figure 1.
Figure 1. Main molecular targets and antigens detected in SARS-Cov2 infection used in diagnosis. Created with BioRender.com. (access on 1 October 2021).
Table 1. Main characteristics of molecular and antigen tests in SARS-CoV-2.
| Test Type |
Advantages |
Disadvantages |
Test Sensitivity % |
Test
Specificity % |
| Test for viral genome |
Accurate tests, identifies mutations in the virus, it tracks disease spread. |
Does not detect viral load, does not detect dynamics of infection or the history of prior infection. |
86.1% |
95.8% |
| Test for viral antigen |
Detects proteins on the viral particle surface. |
Less sensitive than molecular tests and often a molecular test need to confirm the positive result. |
61.7% |
98.2% |
| Faster than molecular tests, less expensive, applicable to large number of samples. |
This entry is adapted from the peer-reviewed paper 10.3390/ijerph182413173