The technology of using metallic iron (Fe0) for in situ generation of iron oxides for water treatment is a very old one. The Fe0 remediation technology has been re-discovered in the framework of groundwater remediation using permeable reactive barriers (PRBs). Despite its simplicity, the improvement of Fe0 PRBs is fraught with difficulties regarding their operating modes. The literature dealing with Fe0 remediation contains ambiguities regarding its invention and its development. The present paper examines the sequence of contributions prior to the advent of Fe0 PRBs in order to clarify the seemingly complex picture.
- Decentralized solutions
- Groundwater remediation
- Historical perspectives
- Lab-scaled investigations
- Porosity loss
- Reactivity loss
- Research and development
- Safe drinking water
- Wastewater treatment
- Zerovalent iron
1. Introduction
The world is facing a problem of continuously decreasing availability of fresh water [1][2][3][4]. This is because natural water resources are progressively polluted with anthropogenic chemicals, including chlorinated hydrocarbons [5][6]. Previous efforts to remediate polluted groundwater have culminated in the development of permeable reactive barriers (PRBs) [7][8][9]. PRBs are subsurface filters filled with appropriate materials to treat through-flowing polluted waters. PRBs containing granular metallic iron (Fe0) have been demonstrated as an economically-feasible, environmentally friendly, and technologically simple approach for groundwater remediation [9][10][2][3][4][11]. In addition, PRBs are applicable to a broad range of chemical species, and are less vulnerable to environmental conditions [11][12].
The development of the Fe0 PRB technology is currently believed to be fraught with two major difficulties: Reactivity loss and permeability loss [11]. Both aspects are inherent to aqueous iron corrosion and occur everywhere, unless appropriate countermeasures are developed [13][14]. Reactivity loss is perceived as the decrease of electron transfer from Fe0 to contaminants over time caused by the formation of an oxide scale on the Fe0 surface (or in its vicinity). On the one hand, a quantitative electron transfer is impaired by the non-conductive nature of the named oxide scale. On the other hand, permeability loss is perceived as filling the pore space of Fe0-based filters mainly by foreign precipitates (e.g., CaCO3) or mixed precipitates (e.g., FeCO3). However, at pH > 4.5, aqueous iron corrosion is a volumetric expansive process [15][16][17], meaning that the very first cause of permeability loss is pore filling with iron oxides and hydroxides [18][19]. Luo et al. [18] have recently demonstrated porosity loss in a Fe0 filter fed by deionised water (no contaminant, no foreign minerals).
The presentation until now demonstrates that the development of the Fe0 PRB technology has been based on considering Fe0 as a reducing agent. This view implies that iron corrosion by water (the solvent) is a side reaction. The net result is an underestimation of the importance of pore filling by solid iron corrosion products (FeCPs). Considering Fe0 as a reducing agent has culminated in the introduction of the electron efficiency concept (EE concept) [20][21][22]. The EE concept aims at optimizing the Fe0 amounts in PRBs in order to avoid material wastage. The EE concept characterizes the redistribution of electrons from Fe0 to dissolved O2, target contaminants, and co-contaminants (e.g., NO3−). The EE concept frontally contradicts the fact that contaminant reductive transformation and Fe0 oxidative dissolution are not simultaneous processes (electrochemical reaction) [23][24][25]. There is thus a need to clarify the root role of Fe0 in PRBs and related filtration systems.
2. Methodology
The literature reviewed herein corresponds to the one published in the peer-reviewed literature prior to the advent of Fe0 PRBs [26][27][28][29]. No systematic review is performed, rather, studies relevant in answering the research questions were selected. Metal recovery with cementation using Fe0 [30][31] and heavy metal removal from industrial wastewaters [32][33] are not considered. The use of Fe0 in organic synthesis [34] is just considered to specify the reaction conditions which do not correspond to environmental conditions. Typically, organic synthesis by metals (including Fe0) occurs in acidic aqueous solutions (pH < 7.0) and at elevated temperatures (e.g., > 30 °C) [34][35][36].
3. Fe0 in Organic Synthesis: The Béchamp Reduction
Reduction of organics with metals has been known for many decades, but there is no commonly accepted theory of the process. A general agreement exists that hydrogen species are involved in these reductive transformations. In these reactions, double-bonds are broken; halogen atoms are replaced by hydrogen or removed entirely with formation of double bonds; while nitrile, thiocyanide, and other nitrogen and sulfur-containing groups are destroyed [36]. The oldest known reaction involving Fe0, and used on an industrial scale is probably the synthesis of aniline after the Béchamp reduction [37][38].
The Béchamp reduction (Béchamp process) implies the chemical reduction of aromatic nitro compounds to amines in the presence of Fe0 and in dilute acid (iron and acid). In the original version of the Béchamp process, nitrobenzene was used to produce aniline in the presence of iron filings or shavings in a dilute hydrochloric acid. It was postulated that reduction was mediated by Fe0 (electrons from the metal body), and that a slightly acidic pH value was needed (optimum 5.5 £pH £6.6) [39]. However, over the years, it was discovered that the reaction was more or less quantitative in organic acids (e.g., HCOOH) and even in NaCl. This implies that Fe2+ ions (stabilized by Cl–) are able to induce the reduction of nitrobenzene. For details, interested readers are referred to annual reviews on “Amination by reduction” published between 1951 and 1961 by Jesse Werner in Industrial and Engineering Chemistry (American Chemical Society) [38]. For the current presentation, it suffices to recall that: (i) The reactants are pre-heated and then passed through a suitable heated reactor, (ii) ferrous salts could also initiate the chemical reduction of aromatic compounds, and (iii) aniline is removed both from the reaction vapors and the bulk solution (not at the Fe0 surface). Moreover, lower aniline recovery was explained by its occlusion in the matrix of solid iron corrosion products (FeCPs) as the final pH values were constantly higher than 5.0 [39][40].
Summarizing, the century-old Béchamp reduction reveals that organics can be quantitatively reduced by Fe2+ ions in the bulk solution, but at elevated temperatures (e.g., > 50 °C). In other words, Fe0 is “just” a generator of Fe2+ for the reductive transformation of nitrobenzene, and the reaction is possibly catalyzed by Fe0 and solid FeCPs (e.g., Fe(OH)2, Fe(OH)3), and the reaction products are quantitatively available in the bulk solution. In the Fe0 remediation technology, pollutants (e.g., nitrobenzene) and reaction products (e.g., aniline) must be removed from the aqueous phase. This is particularly true for safe drinking water provision. However, as a rule, in the concentration range of natural waters, chemical reduction is not a contaminant removal mechanism. In fact, the residual concentrations of the parent chemical and reaction products as per the equilibrium constant, are larger than the maximum permissible contamination level in most of the cases [41].
4. Fe0 for Safe Drinking Water Provision
In his historical textbook on water treatment, Davis [42] highlighted the following materials as potentially suitable for safe drinking water provision in filtration systems: Animal charcoal, bricks, carbonide of iron, coke, compressed sponge, porous tiles, sand, spongy iron, unglazed earthenware, and wood charcoal. From this list, two materials are Fe0-based: Carbonide of iron and spongy iron. Spongy iron was explicitly described for its capacity for "removal and destruction of organic matter". Filtration systems are often operated under ambient conditions (about 20 °C) and without pH adjustment. In other words, as early as the end of the 19th century, Fe0 was used for the abiotic or chemical destruction of organic matter and nitrates under environmental conditions [43]. Remember that pioneers of the Fe0 PRBs have traced this process back to the 1970s. Admittedly, organics of concern were not halogenated carbons, but the ancient literature on water treatment using the Fe0 system remains largely unexploited [44]. The three known ancient Fe0-based systems for safe drinking water provision will be briefly presented in this section.
4.1. The Bischof Process
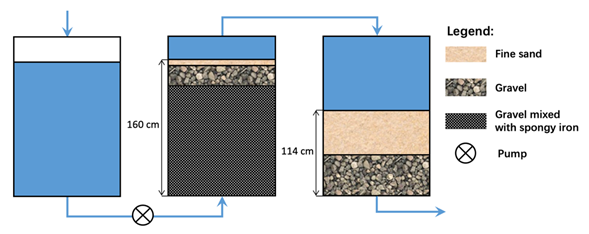
In 1871, Prof. Gustav Bischof (Glasgow) patented a system for water treatment at the household level using spongy iron as a reactive material [45][46]. Porous spongy iron or sponge iron corresponds to direct reduction of iron and was the best innovation in efforts to use Fe0-materials in a decentralized water treatment as summarized by Mwakabona et al. . The Bischof process was then tested and used for the water supply of the city of Antwerp (Belgium) (10,000 m3 d–1) between 1881 and 1883 [47]. The Bischof process (Figure 1) could efficiently supply the city with safe drinking water for 18 months without any perturbation or need for maintenance. However, after 18 months, the filters experienced clogging and could no longer produce enough water to cover the needs of the 200,000 inhabitants. Hence, it became necessary to adopt a more rapid system: The revolving purifier or Anderson process [47][48].
A careful examination of the origin of filter clogging clearly traced it to the Fe0/gravel layer (Figure 1) . In this layer, gravel and Fe0 particles were cemented to a compact mass which locally reduced the interconnectivity of available pore spaces and the hydraulic conductivity (permeability) of the whole filter. The rationale for the use of the volumetric Fe0/gravel ratio of 1/3 (25% Fe0) was not given in Davis and could not be found in the original works of Bischof . The volumetric expansive nature of iron corrosion was also not yet discovered by then. However, the implementation of the Bischop process in Antwerp had clearly demonstrated that Fe0-based filters are prone to clogging caused by iron corrosion. Since the Fe0 proportion matters, it can be postulated that higher Fe0 ratios (e.g., 50, 75, or 100%) would have yielded less sustainable Fe0 filter systems (less than 18 months of service life).
Figure 1. Schematic diagram of the Bischof’s spongy iron filter as described by Davis [42]. Arrows show the direction of water flow.
4.2. The Anderson Process
In 1885, Anderson patented the “revolving purifier” and used it in Antwerp to replace the spongy iron filters . The revolving purifier entails vigorously churning up the polluted water for up to 5 min with Fe0 filings or shavings in a cylinder. During this time, iron hydroxides precipitate and occlude contaminants, including organic matters. The flocs are then removed in a subsequent filtration on gravel and sand. In other words, the Anderson process roughly corresponds to coagulation/flocculation, wherein flocs are not generated by iron salts but are in situ produced from Fe0. It is obvious that the Bischof process relies on the same principles, with the subtle but important difference that iron precipitation occurs in the vicinity of Fe0 particles and not in the bulk solution. The subsequent Emmons process intuitively used this evidence. The Anderson process could produce up to 20,000 m3 d–1 in Antwerp.
4.3. The Emmons Process
Around 1950, the US Atomic Energy Commission initiated research to thoroughly investigate the decontamination of water polluted with radionuclides at a decentralized level. The tested methods included adsorption, coagulation, distillation, and ion exchange. While investigating adsorptive methods, Lauderdale and Emmons [49] found that steel wool (Fe0 SW) was capable of quantitatively removing radioactivity from the aqueous phase. This observation led to the investigation of powdered metals (e.g., Al0, Cu0, Fe0, Zn0) as an alternative to Fe0 SW for the removal of radioactivity from water [50].
The patented Emmons process [51] was a promising water treatment technology for decentralized safe drinking water provision. It entails using a mixed bed ion exchange in conjunction with another bed filled with Fe0 SW, clay, and activated carbon. However, the systems were outcompeted by pure ion exchange systems, partly because of its selectivity towards negatively charged radionuclides [52][53]. The merit of the Emmons process was to reiterate the crucial importance of permeability loss in Fe0-based filters, while revealing the importance of multi-barrier systems to account for the specificities of individual contaminants.
The presentation of the ancient Fe0 technology for safe drinking water demonstrates that already in the 1950s, Fe0 and other elemental metals (e.g., Al0, Cu0, Fe0, Zn0) were demonstrated as powerful reactive materials for the removal of nitrate, organic substances, pathogens, and radionuclides from polluted waters. The corresponding filtration systems, working under ambient conditions (e.g., O2 level, temperature), were plagued by permeability loss certainly due to solid FeCPs. However, the volumetric expansive nature of iron corrosion as demonstrated by Pilling and Bedworth was not considered in solving the clogging problem. Instead, Lauderdale and Emmons used a grade 0 (d = 50 mm) Fe0 SW and suggested the use of coarser Fe0 SW or granular Fe0 (d > 50 mm) to avoid (or delay) clogging. Oldright et al. partly justified permeability loss of Fe0 filters by larger Pb2+ ions replacing Fe2+ in filters. This plausible argument has equally not considered the volumetric expansion of Fe.
5. Fe0 for Agricultural Wastewater
The need for an affordable solution for wastewater treatment, particularly water containing high levels of phosphate is a classic example of how technology can be rediscovered in different contexts. For example, Section 4 has already pointed out how the Emmons process was discovered independently from the Bischof process. In 1992, while seeking for applicable and cost-effective solutions for phosphate removal from wastewaters, George Frigon suggested Fe0 SW as a good material to in situ generate “oxides” for phosphate removal [54]. In this communication, this tool will be operationally termed the Frigon process. One key advantage of the Frigon process is that, Fe0 SW is readily available and is acceptable “from an engineering viewpoint”. In 2007, Andrew J. Erickson independently presented the Frigon process [55] where sand was used as admixing material instead of peat similar to the Frigon process. The Erickson process is particularly interesting because it was introduced more than a decade after the advent of the Fe0 PRB technology, but was introduced as a stand-alone technology. Only in the further development of the Erickson process was the knowledge from the Fe0 PRB considered [56][57]. For example, it was suggested that Fe0 SW was replaced by the granular Fe0. Another interesting feature of the Erickson process is that, it used only less than 5% by weight of Fe0 SW and was efficient for years without any clogging problems[56].
Another field of application where Fe0 presented promising results is that of Se removal from agricultural drainage water. Filtration on Fe0 beds was investigated as a feasible, cost-effective, and practical alternative to biological precipitation, flow-through wetlands, ion-exchange, microalgal-bacterial treatment, reverse osmosis, solar ponds, and volatilization [58]. In 1985, the Harza Engineering Company tested a pilot-scale process using iron filings in flow-through beds to remove Se from agricultural drainage water (Harza process) [59]. Se removal was quantitative, but the testing was discontinued because the columns quickly cemented with precipitates (FeCPs). It was first postulated that Fe0 reduces SeVI to SeIV and Se0. Further studies conclusively demonstrated that Se was not reduced by Fe0 (no electrochemical mechanism), but rather, by FeII species generated in situ [60]. Anderson [60] also demonstrated that Se was removed by adsorption onto and co-precipitation with FeCPs, despite the observed chemical reduction. The tested filter beds contained 100% Fe0 and were very efficient at removing Se, but were not sustainable due to clogging. Testing the Harza process has demonstrated that Fe0 filtration can decrease Se concentrations to very low values and suggested that the Harza process “might be useful as a polishing step following microbial treatment” [58]. However, the Erickson process suggests that decreasing the Fe0 proportion in the beds (e.g., 25% v/v) would make the Harza process a stand-alone sustainable technology for selenium removal.
6. Fe0 for Domestic and Industrial Wastewaters
Previous sections have demonstrated the ability of Fe0 filters to treat water polluted with organic matter, phosphate, and nitrate, which are three main components of human wastes. Thus, Fe0 filters are also a good candidate for the decentralized treatment of domestic wastewaters. Conventional methods for decentralized domestic wastewater treatment include lagoons, sand filters, and wetlands. However, these technologies presented numerous drawbacks, such as evaporation of huge quantities of valuable water, generation of significant odor, and high demand for land. Additionally, their treatment performance depends on seasonal variations and require frequent maintenance operations .
In 1993, Wakatsuki et al. [61] presented in the English peer-reviewed literature a system first published between 1989 and 1991 in Japanese (Jpn. J. Soil Sci. Plant Nutri.). Then, the new Fe0-based wastewater treatment technology is termed as a multi-soil-layering (MSL) system. In a MSL system, Fe0 in soil is oxidized to ferrous ions which in situ coat the available surface (e.g., in zeolite layers), and are oxidized further to ferric iron which can fix phosphate ions. Fe0 oxidation consumes oxygen and contributes to the development of anaerobic conditions. The MSL system has been successfully tested for domestic wastewater treatment in several countries over the past 30 years [62][63][64]. Compared to the other alternatives discussed earlier, the MSL technology is very cost effective and has an effective service life estimated to be more than 20 years. The MSL system has several advantages, including (i) occupies a small area, (ii) has a high hydraulic capacity, (iii) simple maintenance and no frequent clogging, and (iv) requires no energy [62]. Therefore, the MSL system has the potential to become a sustainable domestic wastewater treatment option in low-income communities in the developing countries. MSL are very flexible systems which can be selectively designed with available materials. Their huge potential in achieving universal sanitation cannot be overemphasized.
The very last important aspect of the ancient Fe0 literature is the concept for wastewater treatment presented in 1991 by Michael Boris Khudenko. Khudenko suggested the use of cementation using Fe0 as a tool to reductively degrade organics in wastewaters. Clearly, a copper salt (e.g., CuSO4) was used to oxidize Fe0 (Equation (1)) to produce FeII species, which in turn reduce organics in a parallel (not simultaneous) reaction (Equation (2)). Contaminant degradation is optimal at lower pH values (H+ consumption), and its extent depends on the Cu2+ concentration, among other parameters. The Fe0 PRB literature has mostly considered that contaminants are reduced by Fe0 (Equation (3)), while Fe0 corrosion by water (Equation (4)) has been regarded as a side reaction.
|
Fe0 + Cu2+ = Cu0 + Fe2+ |
|
2 Fe2+ + RCl + H+ = 2 Fe3+ + RH + Cl− |
|
Fe0 + RCl + H+ = Fe2+ + RH + Cl− |
|
Fe0 + 2 H+ = Fe2+ + H2 |
The most trivial argument against Equation (3) is the presence of a non-conductive oxide scale shielding the Fe0 surface, hence electron transfer from Fe0 to the contaminant is impossible [65]. On the other hand, chemical reduction according to Equation (3) was documented in the Béchamp process (Section 3). In other words, before the mechanistic discussion initiated by Matheson and Tratnyek in the framework of research for Fe0 PRBs, the scientific literature had already presented evidence that at a pH value of natural waters, contaminants are quantitatively removed in well-designed Fe0/H2O systems. As demonstrated herein, Fe0 is corroded by water (Equation (4)), while contaminants are reduced in parallel reactions (Equation (2)).
This entry is adapted from the peer-reviewed paper 10.3390/pr8080977
References
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R. Tchobanoglous, In Principles of Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 674.
- Henderson, A.D.; Demond, A.H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review. Environ. Eng. Sci. 2007, 24, 401–423.
- Gheju, M. Hexavalent chromium reduction with zero-valent iron (ZVI) in aquatic systems. Water Air Soil Pollut. 2011, 222, 103–148.
- Ghauch, A. Iron-based metallic systems: An excellent choice for sustainable water treatment. Freiberg Online Geosci. 2015, 32, 1–80.
- Detay, M.; Alessandrello, E.; Come, P.; Groom, I. Groundwater contamination and pollution in micronesia. J. Hydrol 1989, 112, 149–170.
- Mackay, M.D.; Cherry, J.A. Groundwater contamination: Pump-and-treat remediation. Environ. Sci. Technol 1989, 23, 630–636.
- McMurty, D.C.; Elton, R.O. New approach to in-situ treatment of contaminated groundwaters. Environ. Progr 1985, 4, 168–170.
- Starr, R.C.; Cherry, J.A. In situ remediation of contaminated Ground water: The funnel-and-Gate System. Ground Water 1994, 32, 465–476.
- O’Hannesin, S.F.; Gillham, R.W. Long-term performance of an in situ "iron wall" for remediation of VOCs. Ground Water 1998, 36, 164–170.
- Lee, G.; Rho, S.; Jahng, D. Design considerations for groundwater remediation using reduced metals. Korean J. Chem. Eng. 2004, 21, 621–628.
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248.
- Richardson, J.P.; Nicklow, J.W. In situ permeable reactive barriers for groundwater contamination. Soil Sediment Contam. 2002, 11, 241–268.
- Bartzas, G.; Komnitsas, K. Solid phase studies and geochemical modelling of low-cost permeable reactive barriers. J. Hazard. Mater. 2010, 183, 301–308.
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180.
- Pilling, N.B.; Bedworth, R.E. The oxidation of metals at high temperatures. J. Inst. Metals 1923, 29, 529–591.
- Caré, S.; Nguyen, Q.T.; L'Hostis, V.; Berthaud, Y. Mechanical properties of the rust layer induced by impressed current method in reinforced mortar. Cement Concrete Res. 2008, 38, 1079–1091.
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. CLEAN—Soil Air Water 2013, 41, 275–282.
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–2351.
- Domga, R.; Togue-Kamga, F.; Noubactep, C.; Tchatchueng, J.B. Discussing porosity loss of Fe0 packed water filters at ground level. Chem. Eng. J. 2015, 263, 127–134.
- Liu, H.; Wang, Q.; Wang, C.; Li, X.-Z. Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem. Eng. J. 2013, 215–216, 90–95.
- He, F.; Gong, L.; Fan, D.; Tratnyek, P.G.; Lowry, G.V. Quantifying the efficiency and selectivity of organohalide dechlorination by zerovalent iron. Environ. Sci. Process. Impacts 2020, 22, 528.
- Gillham, R.W. Development of the granular iron permeable reactive barrier technology (good science or good fortune). In Advances in Environmental Geotechnics, Proceedings of the International Symposium on Geoenvironmental Engineering in Hangzhou, China, 8–10 September, 2007; Chen, Y., Tang, X., Zhan, L. Eds.; Springer: Berlin, Germany; London, UK, 2008; pp. 5–15.
- Noubactep, C. Flaws in the design of Fe(0)-based filtration systems? Chemosphere 2014, 117, 104–107.
- Noubactep, C. Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 2016, 153, 528–530.
- Xiao, M.; Hu, R.; Cui, X.; Gwenzi, W.; Noubactep, C. Understanding the operating mode of Fe0/Fe-sulfide/H2O systems for water treatment. Processes 2020, 8, 409.
- Gillham, R.W.; O’Hannesin, S.F. Enhanced degradation of halogenated aliphatics by zero-valent iron. Ground Water 1994, 32, 958–967.
- Lipczynska-Kochany, E.; Harms, S.; Milburn, R.; Sprah, G.; Nadarajah, N. Degradation of carbon tetrachloride in the presence of iron and sulphur containing compounds. Chemosphere 1994, 29, 1477–1489.
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. Environ. Sci. Technol. 1994, 28, 2045–2053.
- Schreier, C.G.; Reinhard, M. Transformation of chlorinated organic compounds by iron and manganese powders in buffered water and in landfill leachate. Chemosphere 1994, 29, 1743–1753.
- Gould, J.P.; Escovar, I.B.; Khudenko, B.M. Examination of the zinc cementation on cadmium in aqueous solutions. Water Sci. Technol. 1987, 19, 333–344.
- Noubactep, C. Elemental metals for environmental remediation: Learning from cementation process. J. Hazard. Mater. 2010, 181, 1170–1174.
- Oldright, G.L.; Keyes, H.E.; Miller, V.; Sloan, W.A. Precipitation of lead and copper from solution on sponge iron. BuMines B 1928, 281, 1–131.
- Gould, J.P. The kinetics of hexavalent chromium reduction by metallic iron. Water Res. 1982, 16, 871–877.
- March, J. Advanced Organic Chemistry: Reaction Mechanism and Structure; McGraw-Hill Book Co: New York, NY, United States, 1968.
- Werner, J. Amination by reduction. Unit Processes Review. Ind. Eng. Chem. 1961, 53, 77–78.
- Khudenko, B.M. Feasibility evaluation of a novel method for destruction of organics. Water Sci. Technol. 1991, 23, 1873–1881.
- Béchamp, A. De l’action des protosels de fer sur la nitronaphtaline et la nitrobenzine: Nouvelle méthode de formation des bases organiques artificielles de Zinin. Ann. Chim. Phys. 1854, 42, 186–196.
- Werner, J. Amination by reduction. Ind. Eng. Chem. 1951, 43, 1917–1919.
- Popat, V.; Padhiyar, N. Kinetic study of Bechamp process for p-nitrotoluene reduction to p-toluidine. Int. J. Chem. Eng. Applications 2013, 4, 401–405.
- Noubactep, C. Relevant reducing agents in remediation Fe0/H2O systems. Clean—Soil Air Water 2013, 41, 493–502.
- Kochenov, S.V.; Korolev, K.G.; Dubinchuk, V.T.; Medvedev, Y.L. Experimental data on the conditions of precipitation of uranium from aqueous solutions. Geokhimiya 1977, 11, 1711–1716.
- Davis, F. An Elementary Handbook on Potable Water; Silver, Burdett & Co: New York, NY, USA; Boston, MA, USA; Chicago, IL, USA, 1891; p. 118.
- Hatton, F. On the oxidation of organic matter in water by filtration through various media; and on the reduction of nitrates by sewage, spongy iron, and other agents. J. Chem. Soc. Trans. 1881, 39, 258–276.
- Mwakabona, H.T.; Ndé-Tchoupé, A.I.; Njau, K.N.; Noubactep, C.; Wydra, K.D. Metallic iron for safe drinking water provision: Considering a lost knowledge. Water Res. 2017, 117, 127–142.
- Bischof, G. The Purification of Water: Embracing the Action of Spongy Iron on Impure Water; Bell and Bain: Glasgow, UK, 1873; pp. 1–19.
- Bischof, G. On putrescent organic matter in potable water. Proc. R. Soc. Lond. 1877, 26, 258–261.
- Devonshire, E. The purification of water by means of metallic iron. J. Frankl. Inst. 1890, 129, 449–461.
- Anderson, W. On the purification of water by agitation with iron and by sand filtration. J. Soc. Arts 1886, 35, 29–38.
- Lauderdale, R.A.; Emmons, A.H. A method for decontaminating small volumes of radioactive water. J. Am. Water Work. Assoc. 1951, 43, 327–331.
- Lacy, W.J. Removal of radioactive material from water by slurrying with powdered metal. J. Am. Water Work. Assoc. 1952, 44, 824–828.
- Emmons, A.H.; Lauderdale, J.R.A. Process for Water Decontamination. US Patent 2,752,309, 26 June 1956.
- Swope, H.G. Mixed bed ion exchange for the removal of radioactivity. J. Am. Water Work. Assoc. 1957, 49, 1085–1102.
- Levin, H.; Diamond, W.J.; Brown, B.J. Influence of Ionic Strength on ion Exchange. Ind. Eng. Chem. 1959, 51, 313–318.
- James, B.R.; Rabenhorst, M.C.; Frigon, G.A. Phosphorus sorption by peat and sand amended with iron oxides or steel wool. Water Environ. Res. 1992, 64, 699–705.
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497.
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042.
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672.
- Frankenberger, W.T.; Amrhein, C.; Fan, T.W.; Flaschi, D.; Glater, J.; Kartinen, E.; Kovac, K.; Lee, E.; Ohlendorf, H.M.; Owens, L. Advanced treatment technologies in the remediation of seleniferous drainage waters and sediments. Irrig. Drain. Syst. 2004, 18, 19–41.
- Harza Engineering Co. Selenium Removal Study. Report to Panoche Drainage District; Harza Engineering Co.: Firebaugh, CA, USA, 1986.
- Anderson, M.A. Fundamental aspects of selenium removal by Harza process. In Rep San Joaquin Valley Drainage Program; US Department Interior: Sacramento, CA, USA, 1989.
- Wakatsuki, T.; Esumi, H.; Omura, S. High performance and N, P removable on-site domestic wastewater treatment system by multi-soil-layering method. Water Sci. Technol. 1993, 27, 31–40.
- Latrach, L.; Ouazzani, N.; Hejjaj, A.; Mahi, M.; Masunaga, T.; Mandi, L. Two-stage vertical flow multi-soil-layering (MSL) technology for efficient removal of coliforms and human pathogens from domestic wastewater in rural areas under arid climate. Int. J. Hyg. Environ. Health 2018, 22, 64–80.
- Masunaga, T.; Sato, K.; Zennami, T.; Fujii, S.; Wakatsuki, T. Direct treatment of polluted river water by the multi-soil-layering method. J. Water Environ. Technol. 2003, 1, 97–104.
- Chen, X.; Luo, A.C.; Sato, K.; Wakatsuki, T.; Masunaga, T. An introduction of a multi-soil-layering system: A novel green technology for wastewater treatment in rural areas. Water Environ. J. 2009, 23, 255–262.
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water treatment using metallic iron: A tutorial review. Processes 2019, 7, 622.