Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Psychology, Biological
Growth hormone (GH) exerts multiple effects on different organs including the kidneys, either directly or via its main mediator, insulin-like-growth factor-1 (IGF-1).
- growth hormone
- insulin-like growth factor 1
- growth hormone receptor
1. Introduction
Most animals must undergo a transition from maternal environment to independent life through processes of growth and maturation. Important hormonal regulators of childhood growth are growth hormone (GH), insulin-like growth factor 1 (IGF1), sex steroids, and thyroid hormone. GH and IGF1 are part of an axis, which is essential for bone and organs growth. The kidneys express both GH as well as IGF1 receptors, and are one of the key target organs for these hormones’ actions. This review concentrated on the roles of these hormones in physiological and pathological kidney conditions.
2. Normal GH-IGF1 Axis and Physiology
GH is produced by somatotroph cells of the anterior pituitary and secreted in a pulsatory way under the positive control of hypothalamic GH-releasing hormone (GHRH) and the negative control of somatostatin [1]. The response to GHRH is mediated via GH-releasing hormone receptor (GHRHR), a G protein–coupled receptor (GPCR) expressed specifically in somatotrophs [2]. Other factors such as insulin-like growth factor (IGF1), neuropeptide Y, and hyperglycemia inhibit GH secretion, and hypoglycemia, thyroxine, ghrelin, klotho, and glucocorticoids stimulate GH secretion [3].
GH acts by binding to GH receptor (GHR) to stimulate, among other genes, the synthesis of insulin-growth factor-1 (IGF1). The bioavailability of GH is regulated by GH-binding protein (GHBP), which is the extracellular part of GHR. Intracellular signal transduction after GH binding to its receptor requires the activation of Janus-associated kinase 2 (JAK2) [4], which stimulates phosphorylation of signal transducer and activator of transcription (STAT) proteins MAPK and PI3K. STAT proteins migrate to the nucleus, activating, among others, gene transcription of IGF1, the main mediator of GH action. In addition, suppressors of cytokine signaling (SOCS) are activated, which dephosphorylate STAT, leading to a negative feedback action on GH [5]. Circulating IGF1 suppresses pituitary GH secretion in a negative feedback loop. IGF1 is synthesized mostly in the liver, but also in peripheral tissues under GH regulation, although nutrition, insulin, thyroid, and sex hormones also affect its expression [6]. The effects of IGF1 are mediated by the type 1 IGF receptor (IGF1R) in a signaling pathway similar to insulin/insulin receptor (IR). IGF1R and IR share amino acid identity, and can be activated both by insulin, IGF1, and IGF2. [7]. IGF1R is a membrane-bound tyrosine kinase heterotetramer, and its activation leads to autophosphorylation of tyrosine residues, leading to signal transduction [8]. The bioactivity of circulating IGF1 is modulated by IGF-binding proteins (IGFBPs 1-6), which facilitate its stability in serum and extracellular matrices. Most IGFs in serum are bound to IGFBP and the acid-labile subunit (ALS), a protein that stabilizes IGF [9,10], and this complex serves as reservoir of IGFs, keeping serum concentration of free IGFs constant. Plasma concentration of IGFBP3 and ALS are also increased by GH, similar to IGF1.
3. GH-IGF1: Axis or Independent Functions?
Whereas GH is only synthesized in pituitary, GHR and IGF1 are expressed in many tissues including the kidneys. Originally GH action was thought to be mediated only through IGF1, called somatomedin, without any direct effects (“somatomedin theory”) [11]. Later, “dual effector hypothesis” suggested that GH also acts directly to promote cell differentiation, independent of IGF1 [12,13,14]. Concentrating on kidneys as one of the target organs for both GH and IGF-1, GH treatment increased kidney IGF1 mRNA levels in hypophysectomized rats, confirming local renal IGF1 production [15]. IGF1 levels are higher in renal venous blood than in renal arterial blood, suggesting significant renal IGF1 biosynthesis [16]. Evidence for a direct IGF1 action in the kidney also comes from studies showing that prolonged treatment with recombinant human (rh) IGF1 increased kidney size in hypophysectomized rats [17] and enhanced the glomerular filtration rate (GFR) in healthy men [18].
4. Observations from Knockout and Transgenic Animals
Animal models of gene inactivation, as well as pathophysiological models, provide important data on mechanisms and role of GH/IGF1 in renal organogenesis. Evidence of both dependent and independent functions of GH and IGF1 on the kidney come from genetically engineered animal models (see Table 1 and Table 2). Examples of the knockout models, where mutations were introduced in every step of the axis (GHRH → GH → GHR/GHBP → JAK2 → STAT5 → IGF1 → IGF1R), followed by the transgenic models, overexpressing genes along this axis, are discussed here.
Biallelic mutation in GHRH causes isolated growth hormone deficiency due to impaired GH secretion in anterior hypophysis [2].
GH knockout mice (GH −/−), which show no circulating GH, also show disproportionally reduced kidney weight compared with wild-type mice, even after correction for reduced body weight [19].
GHR/GHPB knockout mice lack functional GH receptors and exhibit GH resistance manifested by decrease in circulating IGF1 levels and growth retardation, starting later after birth. These mice also have disproportionally small kidneys [20].
Germline deletion of Jak2 (downstream of GHR, but also of other hormones and cytokines) in mice resulted in embryonic lethality due to a lack of hematopoiesis [21]. Homozygous mutation in the gene for STAT5 resulted in IGF-1 deficiency and growth hormone insensitivity, indicating impaired postreceptor signaling for GH. It leads to abnormal postnatal growth, facial dysmorphism, and markedly reduced serum concentrations of IGF-1, IGFBP-3, and acid-labile subunit, and immunodeficiency [22]. The latter seems to be due to the importance of both JAK2 and STAT5 not just in mediating GH signals, but also other cytokines involved in immune as well as hematopoetic regulation, such as the erythropoietin receptor [23]. STAT5 knockout mice died perinatally, and 1–2% of survivors were dwarf, with anemia and immunodeficiency [24].
IGF1 knockout mice have severe growth retardation, deficiencies in bone and muscle development, infertility, and lethal respiratory failure due to lung hypoplasia, highlighting the importance of GH/IGF1 axis in different tissues development. Their kidneys are proportionally small with decreased glomerular size and nephron number [25,26].
Prenatal IGF1R knockout embryos exhibit growth retardation and generalized developmental abnormalities, comprising hypoplasia, altered central nervous systems, abnormal skin formation, delayed bone development, reduced pancreatic beta-cells, failure of testicular determination, lung immaturity, and cochlear defects [27]. As IGF1R is closely related to the IR, partly sharing amino acid identity, increased IGF2–mediated IR signaling can rescue mouse embryonic development to prevent dwarfism in IGF1R knockout mice [28].
Mice with homozygous null mutations in Igf1r had normal embryonic development but had low weight and died soon after birth, whereas heterozygous mice had normal growth up to the weaning period, followed by a significant reduction in weight gain and development of insulin resistance [29]. Therefore, the phenotype of the knockout animal is more severe as the location of the affected gene is more distal along the signaling pathway, as described for example for other pathways where the kidneys are a target organ [30].
Excessive GH levels are associated with renal hypertrophy in humans and rodents [31]. Transgenic mice overexpressing the GH gene exhibit excessive GH and IGF1 concentrations, resulting in a giant phenotype and organomegaly, including increased kidney weight even when related to increased body weight [32]. These animals also develop glomerulosclerosis and kidney failure, in association with glomerular hypertrophy and progressive albuminuria [33]. Transgenic mice overexpressing IGF1 are larger than wild-type mice, have proportionately enlarged kidneys [34], and also show glomerular hyperthrophy, but do not develop glomerulosclerosis [35,36]. These findings indicate that GH excess causes glomerular and podocyte hypertrophy sufficient to induce glomerulosclerosis independently of IGF1.
Consistent with the role of IGFBPs as inhibitors of IGF action, their generalized overexpression predominantly results in growth retardation. Mice engineered to overexpress IGFBP-1 have prenatal and postnatal growth retardation, disproportionally small brains, splenomegaly, and glucose intolerance. Their kidneys are proportionally small with a decreased nephron number; they also develop glomerulosclerosis without glomerular hypertrophy [38,39,40,41]. Transgenic mice that overexpress IGFBP-2 have only mild growth retardation, with proportionally small kidneys [42]. Mice overexpressing IGFBP-3 have selective organomegaly (spleen, liver, heart) [43], and disproportionally small kidneys [44], whereas those overexpressing a mutant of IGFBP-3 with impaired IGF binding have normal postnatal growth and kidney size [45], suggesting that the effects on the kidney seen in the former are due to inhibition of IGF actions. IGFBP-4 overexpression in various tissues in mice resulted in hypoplasia of the affected tissue, suggesting a common action in different cell types [37]. Interestingly, only few or no phenotypic changes were observed when separately knocking out each specific IGFBP [46,47,48,49,50].
In the 5/6 nephrectomy mouse model of chronic kidney disease, silencing of SOCS2, a negative regulator of GH action, was shown to overcome CKD-related growth retardation without worsening kidney function. This was explained by elevation of inflammatory cytokines in uremic mice and upregulation of SOCS3, another regulator of cytokine signaling, leading to the prevention of renal GHR overstimulation [51].
5. GH and IGF1 in Normal Renal Development
The GH/IGF1 system plays a key role in normal kidney development, although it does not impair basic kidney formation mediated by the branching morphogenesis process [54]. During embryogenesis, GHR mRNA was detected in rat kidneys from embryonic day 20 and was mainly expressed in the proximal tubules [55]. In the human fetal kidney, GHR-specific immunostaining was shown as early as 8.5 to 9 weeks and most renal tubular epithelial cells became positive by week 13. The staining was stronger in the outer medulla than in the cortex and remained similar at midgestation and after birth. Weak staining was also found in immature glomeruli in early gestation, but disappeared at later developmental stages, suggesting specific GH involvement in glomerular morphogenesis [56].
6. GH/IGF1 Effects on Normal Tubular and Glomerular Functions
Normal kidney function includes glomerular filtration and tubular secretion and reabsorption, leading to fluid and electrolyte balance. In addition, kidneys control blood pressure, as well as hormonal synthesis (such as EPO and active Vitamin D).
GH and IGF1 deficient patients have reduced glomerular filtration rate (GFR) and renal perfusion flow (RPF) [63,64]. Hypophysectomy in humans leads to a rapid decrease in GFR [65], and rhGH treatment leads to GFR and RPF improvement in a dose and time-dependent manner [63,64]. In a cohort of GH-deficient children (isolated or multiple pituitary), GFR was in normal physiological levels but lower than in controls and significantly increased after 3 years of rhGH in parallel to kidney and body growth [66]. In contrast, acromegalic patients have increased GFR and RPF [65,67] and albuminuria [68,69,70] compared with healthy subjects.
GH and IGF1 are involved in tubular handling of sodium, water, calcium, and phosphate, and are also known to regulate tubular gluconeogenesis [76]. GH deficiency is associated with reduced sodium and total body water content [77], and rhGH-replacement therapy improves these parameters [78]. Treatment with high rhGH doses may even lead to acute fluid retention [79]. In contrast to that, acromegalic patients show an increase in total body water and sodium and may present with edema. Treatment of GH-producing tumors reverses these changes [80,81].
The direct, IGF-1-independent effect of GH on sodium and fluid retention is controversial: infused recombinant IGF1 did not change body weight and sodium excretion in healthy subjects [18,82], but treatment with rhIGF1 improved hydration status in children with GH insensitivity due to GHR inactivating mutations, indicating that sodium and water retaining properties of GH are at least partly mediated by IGF1 [83].
Liver-specific deletion of the IGF1 gene increased urinary sodium and potassium excretion [84], confirming the role of IGF1 in water and sodium handling. Evidence for both direct GH/IGF1 action on kidney tubule and indirect mechanisms involving the renin-angiotensin-aldosterone system (RAAS) or natriuretic peptides exists. Rapid increase in plasma renin activity and aldosterone level after rhGH administration in healthy men was reported [85], and treatment with angiotensin converting enzyme (ACE)-inhibitor captopril and mineralocorticoid receptor antagonist spironolactone abolished the GH-induced increase in extracellular volume [86]. Decrease of atrial natriuretic peptide concentration after rhGH treatment was also shown [87]. Recent data show evidence for direct action of GH and IGF1 on epithelial sodium channels (controlled by aldosterone) in cortical collecting ducts [88]. Reversal of GH/IGF1 excess in acromegalic patients decreases ENaC activity [89]. In rats with GH-secreting tumors, the direct stimulatory effect of excess GH on ENaC-dependent sodium transport in distal nephron was demonstrated. Enhanced natriuretic response after ENaC blocking by amiloride and enhanced Na/K-ATPase activity selectively in the cortical collecting ducts were demonstrated, providing additional evidence for increased sodium reabsorption in the late distal nephron during a chronic GH excess. Changes in ENaC subunit proteins, known to be associated with increased ENaC activities [90], were shown in these rats and were not accompanied by elevated aldosterone levels [88]. In humans, active acromegaly was also associated with an increased response to amiloride, providing evidence of increased renal ENaC activity in excess of GH/IGF1 [89]. Another possible molecular target of GH/IGF1 in the kidney tubule is the sodium-potassium pump Na/K-ATPase. GH has been shown to enhance the hydrolytic activity of Na/K-ATPase in rat kidney [91].
Being the major hormones mediating somatic growth, GH and IGF1 promote positive calcium and phosphate balance, influencing, for example, 1.25 (OH)2 vitamin D synthesis, which is crucial for intestinal calcium absorption. GH stimulation of renal calcitriol synthesis is mediated by IGF1 via induction of 1α-hydroxylase in the proximal tubule [92]. GH-replacement therapy, as well as treatment with rhIGF1, increased serum calcitriol levels in GH-deficient patients [93]. Several studies in GH-deficient adults have shown transient elevation in blood calcium level and urinary calcium excretion during rhGH-treatment [94,95]. In contrast, studies in children showed unchanged or even decreased blood calcium levels during long-term rhGH replacement, probably related to modifications of mineral metabolism and a significant increase in bone density [96].
Long-term rhGH treatment leads to a persistent increase in plasma phosphate concentrations in GH-deficient children [93] and adults [91,92,102], which is mediated by a direct antiphosphaturic action of IGF1 in the proximal tubule [103]. IGF1 directly increases phosphate reabsorption via increase of Na-Pi2a expression in proximal tubule, which could be completely blocked by an anti-IGF1R antibody [104,105]. Patients with acromegaly may have mild hyperphosphatemia that normalizes after treatment of their GH-secreting tumor [106].
The physiologic roles of GH and IGF1 in different nephron segments are depicted in Figure 1.
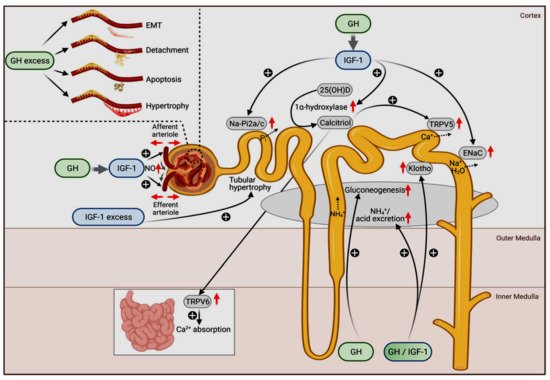
Figure 1. Physiological (main figure) and pathophysiological actions of GH (upper left insert) and IGF-1 on the kidneys. The original figure has been published by Hafner et al. [107] and published here with permission. The figure is licensed under a Creative Commons Attribution 4.0 International License. See link to the Creative Commons license (http://creativecommons.org/licenses/by/4.0/, accessed on 29 November 2021). No changes to the original figure were made.
This entry is adapted from the peer-reviewed paper 10.3390/cells10123371
This entry is offline, you can click here to edit this entry!
