1. Background
In the past decade, the field of cancer immunotherapy has rapidly advanced, establishing a crucial role for immune checkpoint blockers in the treatment of a variety of cancer types. In parallel with these remarkable clinical developments, further efforts have focused on ways of unleashing adaptive immune responses against cancer. CD47, a cell surface molecule overexpressed by several cancer types that facilitates immune escape from macrophages, dendritic cells and natural killer cells, and its ligand SIRPα, have emerged as potential therapeutic targets. A number of agents directed to CD47/SIRPα have been developed and demonstrated preclinical activity. Early phase clinical trials are investigating CD47/SIRPα directed agents with available data, suggesting safety and preliminary activity.
2. Role of CD47/SIRPα in Cancer
In the early 1990s, the first oncological studies of CD47 identified it as a potential tumor marker for ovarian cancer [
25]. This was followed by investigations on a wide variety of solid and hematological cancer types, including head and neck small-cell carcinoma (HNSCC), breast cancer, acute myeloid leukemia (AML), non-Hodgkin’s lymphoma (NHL), myeloma demonstrating differential overexpression of CD47 between cancer cells and matched normal cells [
9,
20,
21,
24,
26,
27,
28,
29].
The role of the CD47/SIRPα interaction in providing an escape mechanism for cancer cells from macrophage targeting has been well described. Human-derived xenograft models for several types of malignancies demonstrated sensitivity to CD47-blocking antibodies. In culture, these antibodies induced the macrophage-mediated phagocytosis of tumor cells [
30,
31,
32,
33,
34,
35,
36,
37]. The impact of the CD47 blockade on macrophage populations within the tumor microenvironment was also studied. In brief, TAMs display different polarization states between M1 macrophages with anti-tumor phenotypes and M2 macrophages with pro-tumor and immunosuppressive phenotypes [
38,
39,
40]. In a human glioblastoma model, anti-CD47 therapy increased M1 macrophages within the tumor. This finding suggests that anti-CD47 therapy may play a role in shifting the phenotype of macrophages toward the anti-tumorigenic M1 subtype [
41]. CD47 signaling also participates in macrophage recruitment into tumors. Weiskopf and colleagues showed that phagocytosis, following anti-CD47 treatment, causes systemic and local secretion of chemokines and cytokines that recruit macrophages into tumors in mice engrafted with small-cell lung cancer (SCLC) cell lines [
30].
Beyond the activation of macrophage-mediated tumor killing, CD47-SIRPα interruption exerts other multidimensional positive effects on the immune response against cancer cells. For example, CD47-SIRPα blockade augments antibody dependent cellular cytotoxicity (ADCC) via the inhibition of SIRPα, expressed on the surface of NK cells [
42,
43]. Kim and colleagues demonstrated that impaired NK cell activity present in HNSCC cell lines overexpressing CD47 could be reversed with anti-CD47 antibodies [
44]. CD47-SIRPα antagonist agents with an intact or even partially inactive Fc portion embedded in their structure may foster anti-tumor activity via antibody opsonization and destruction of target cells through ADCC or antibody-dependent cellular phagocytosis (ADCP) [
45]. In addition, CD47/SIRPα interaction also has roles in tumor cell apoptosis, proliferation and migration [
46,
47,
48]. CD47 inhibition can also negatively impact the function of other CD47 ligands, such as TSP-1 and integrins. These indirect effects may contribute to the anti-tumor and pro-inflammatory activity of CD47 inhibition. Despite contrasting evidence, a growing body of research highlights the role of TSP-1 in cell proliferation, invasion, metastatic potential, and worse survival rates, either through its interaction with CD47 or independently [
49,
50,
51]. Notably, Kamijo and colleagues reported an association between high TSP-1 expression and worse disease-free survival in cutaneous T cell lymphoma patients. TSP-1 was found to be overexpressed in cutaneous T cell lymphoma, and anti-CD47 antibodies led to the inhibition of TSP-1-mediated cell proliferation in vivo [
52].
Preclinical work has suggested a synergy between the cytotoxic agents and the CD47 inhibitors, especially when cytotoxic therapies were introduced prior to CD47-directed therapies. Neoantigens and nucleic acid remnants, produced from dying cancer cells and released into the tumor microenvironment after chemotherapy, may potentiate anti-CD47 activity [
53]. In the context of hematologic malignancies, in vitro studies showed that azacytidine (a standard of care DNA hypomethylating agent used in the treatment of AML) and myelodysplastic syndrome and venetoclax (a B-cell lymphoma-2 inhibitor used in AML), induces the expression of other pro-phagocytic pathway components such as calreticulin and CD47 [
54].
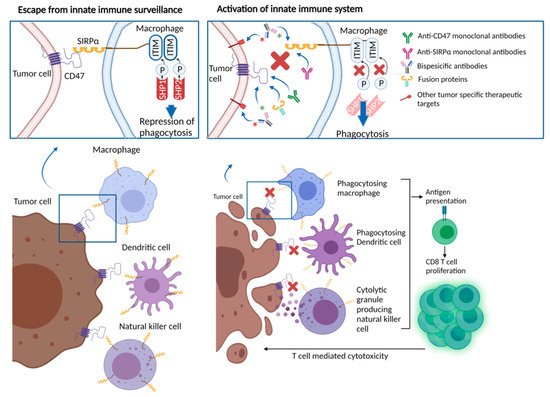
Perhaps more intriguingly, the macrophages involved in phagocytosis function as antigen-presenting cells, linking innate and adaptive immunity [
29,
53,
55]. Thus, targeting the CD47-SIRPα axis, either through the CD47 or SIRPα blockade, may also promote antigen-presenting cell function, and stimulate T cell-mediated anti-cancer immunity (
Figure 1) [
56,
57]. Studies in preclinical models with cancer types including chronic lymphocytic leukemia, colon cancer, melanoma, HNSCC, and glioblastoma, showed the induction of antitumor cytotoxic T cell populations, and reduced regulatory T cell populations in response to anti-CD47 treatment [
26,
55,
58,
59,
60]. These observations were replicated in ex vivo studies. For example, Tao and colleagues assessed tumor samples from esophageal squamous cell cancer patients, showing an inverse relationship between CD8 T cell density and CD47 expression. In mice models with esophageal squamous cell cancer, treatment with anti-CD47 antibodies led to an increase in PD-1 and CTLA-4 expression. Treatment with the combination of CD47, PD-1 and CTLA-4 inhibitors yielded significantly improved survival in mice, compared with anti-CD47 monotherapy or PD-1 and CTLA-4 inhibitor combination, suggesting a rationale for combinatory therapeutic approaches to obtain synergistic effects [
61].
Figure 1. CD47/SIRPα interaction leading to repression of phagocytosis and therapeutic approaches blocking CD47/SIRPα axis.
Clinical implications of CD47 overexpression were also studied in various cancer types with the majority showing an inverse relationship between CD47 overexpression and clinical outcomes [
62]. Chao et al. used flow cytometry and found that NHL cells had two-fold greater CD47 expression than normal germinal center and peripheral blood B cells. Grouping patient samples based on
CD47 mRNA expression levels, investigators showed improved overall survival in patients with CD47 low tumors, especially diffuse large B cell lymphoma (DLBCL), B cell chronic lymphocytic leukemia, and mantle cell lymphoma subsets [
63]. Majeti and colleagues, showed high CD47 expression by gene expression arrays and flow cytometry in leukemia stem cells, compared with normal counterparts in a group of 137 AML patients. Compared with those with low CD47 expression, patients with high CD47 expression had significantly worse overall survival rates (22.1 vs 9.1 months, hazard ratio (HR): 2.02) and event free survival (17.1 vs 6.8 months, HR 1.94) [
32]. Analyzing immunohistochemistry staining of CD47 in bone marrow biopsy samples from 248 AML patients, Galli et al. detected high CD47 staining in one-fourth of the patient samples. Samples with high CD47 staining had higher median blast count, median bone marrow infiltration, and disease burden. Although there was a trend towards unfavorable progression free survival in patients with high CD47, no statistical difference was observed in median progression-free survival, or overall survival [
64]. Melanoma patients with tumors bearing CD47 overexpression were found to have worse overall survival rates and higher rates of distant metastasis [
65]. Similarly, head and neck cancer patients with tumors bearing robust CD47 immunohistochemistry staining had diminished overall survival, compared with those with low CD47 staining [
26]. A study of ovarian cancer demonstrated that increased CD47 expression is associated with worse prognosis, increased migration and invasion, and the induction of epithelial-mesenchymal transition [
66].
5. Therapies Targeting CD47/SIRPα in Cancer
As a result of the promising preclinical data regarding the anti-tumor activity of CD47/SIPRα blockade obtained from in vivo and in vitro studies, several molecules have been developed and are undergoing clinical testing. Functionally, therapeutics under investigation may be classified as (1) CD47 targeting agents, (2) SIRPα targeting agents and (3) bispecific targeting agents.
Table 1 provides a comprehensive list of the ongoing clinical trials of the CD47/SIRPα targeting therapeutics at the time of this publication. Although most of those approaches are currently being tested in early-phase clinical trials to assess safety and tolerability, available data from a number of published studies has revealed promising activity and favorable tolerability. In addition to being tested on their own, trials of combinations with other anti-tumor agents are underway. Inspired by the fact that CD47/SIRPα signaling limits the efficacy of tumor-opsonizing antibodies, a number of clinical trials are evaluating agents targeting this axis in combination with agents such as rituximab, cetuximab and trastuzumab [
30,
63,
67]. Histone deacetylase (HDAC) inhibitors have been shown to enhance checkpoint inhibitor therapy by decreasing immune suppressive cells and increasing tumor antigen presentation [
68,
69]. Given the possible enhancement of tumor immunity, combinations of HDAC inhibitors and CD47 targeting therapies are underway. Other strategies employ a combination of CD47 targeted therapies with immune checkpoint inhibitors and chemotherapies.
Table 1. Clinical trials testing agents targeting CD47/SIRPα axis.
| Agent |
Therapeutic
Target |
Design |
Phase |
Disease Site |
Accrual Goal |
Identifier |
| Monoclonal Antibodies |
| IBI188 (Letaplimab) |
CD47 |
IBI188 +/− rituximab |
I |
Metastatic solid tumors or lymphoma |
92 |
NCT03717103 |
| IBI188 +/− azacitidine |
I |
Myelodysplastic syndrome |
12 |
NCT04485065 |
| Hu5F9-G4 (Magrolimab) |
CD47 |
Hu5F9-G4 (Magrolimab) + Pembrolizumab |
II |
Hodgkin’s lymphoma |
24 |
NCT04788043 |
| Hu5F9-G4 (Magrolimab) |
I |
Hematologic malignancies |
20 |
NCT02678338 |
| Hu5F9-G4 (Magrolimab) + acalabrutinib + rituximab or other combinations without Hu5F9-G4 (Magrolimab) |
I |
Non-Hodgkin’s Lymphoma |
30 |
NCT03527147 |
| Hu5F9-G4 (Magrolimab) + Obinutuzumab + venetoclax |
I |
Non-Hodgkin’s Lymphoma |
76 |
NCT04599634 |
| ZL-1201 |
CD47 |
ZL-1201 |
I |
Metastatic solid tumors or refractory lymphomas |
66 |
NCT04257617 |
| STI-6643 |
CD47 |
STI-6643 |
I |
Metastatic solid tumors |
24 |
NCT04900519 |
| CC-9002 |
CD47 |
CC-90002 +/−rituximab |
|
Part A: Metastatic solid tumors, multiple Myeloma or non-Hodgkin’s lymphoma
Part B, relapsed and/or refractory CD20-positive NHL |
60 |
NCT02367196 |
| AK117 |
CD47 |
AK117 |
I |
Metastatic solid tumors or lymphoma |
162 |
NCT04728334 |
| AK117 + azacitidine |
I/II |
Myelodysplastic syndrome |
190 |
NCT04900350 |
| AO-176 |
CD47 |
AO-176 +/− paclitaxel |
I/II |
Metastatic solid tumors |
132 |
NCT03834948 |
| AO-176 +/− dexamethasone or dexhamethasone + bortezomide |
I |
Multiple myeloma |
102 |
NCT04445701 |
| IMC-002 |
CD47 |
IMC-002 |
I |
Metastatic solid tumors or lymphoma |
24 |
NCT04306224 |
| TQB2928 |
CD47 |
TQB2928 |
I |
Metastatic solid tumors or hematologic malignancies |
20 |
NCT04854681 |
| FSI-189 |
SIRPα |
FSI-189 +/− rituximab |
I |
Non-Hodgkin’s lymphoma (B-cell) |
63 |
NCT04502706 |
| BI 765063 |
SIRPα |
BI 765063 +/− PD-1 inhibitor |
I |
Metastatic solid tumors with SIRPα polymorphism |
116 |
NCT03990233 |
| Bispecific antibodies |
| HX009 |
CD47 and PD-1 |
HX009 |
II |
Metastatic solid tumors |
210 |
NCT04886271 |
| PF-07257876 |
CD47 and PD-L1 |
PF-07257876 |
I |
Non small-cell lung cancer, head and neck squamous cell carcinoma, ovarian cancer |
90 |
NCT04881045 |
| CPO107 (JMP601) |
CD47 and CD20 |
CPO107 (JMP601) |
I |
Non-Hodgkin’s lymphoma (CD-20 positive) |
75 |
NCT04853329 |
| IBI322 |
CD47 and PD-L1 |
IBI322 |
I |
Hematologic malignancies |
182 |
NCT04795128 |
| IBI322 |
Ia |
Metastatic solid tumors |
45 |
NCT04338659 |
| IBI322 |
Ia/Ib |
Metastatic solid tumors |
218 |
NCT04328831 |
| SL-172154 |
SIRPα and CD40L |
SL-172154 (intravenous) |
I |
Ovarian cancer |
40 |
NCT04406623 |
| SL-172154 (intratumoral) |
I |
Head and neck or cutaneous squamous cell carcinoma |
18 |
NCT04502888 |
| TG-1801 |
CD47 and CD19 |
TG-1801 +/− ubitixumab |
Ib |
Hematologic malignancies |
60 |
NCT04806035 |
| IMM0306 |
CD47 and CD20 |
IMM0306 |
I |
Refractory or Relapsed CD20-positive B cell Non-Hodgkin’s Lymphoma |
131 |
NCT04746131 |
| Fusion proteins |
| TTI-622 |
CD47 via SIRPαFc (IgG4) structure |
TTI-622 + rituximab, PD-1 inhibitor, Proteasome inhibitor regimen or rituximab |
Ia/Ib |
Lymphoma or myeloma |
156 |
NCT03530683 |
| ALX148 |
CD47 via SIRPαFc (IgG1) structure |
ALX148 + azacitidine |
I/II |
Myelodysplastic syndrome |
173 |
NCT04417517 |
| ALX148 + venetoclax or azacitidine |
I/II |
Acute myleoid leukemia |
97 |
NCT04755244 |
| ALX148 |
II |
Head and neck squamous cell carcinoma |
112 |
NCT04675333 |
| ALX148 + pembrolizumab |
II |
Head and neck squamous cell carcinoma |
111 |
NCT04675294 |
Major concerns regarding the use of CD47-targeted agents are driven by the ubiquitous expression of CD47, which leads to rapid drug elimination, “antigen sink” and hematologic toxicity, such as anemia and thrombocytopenia [
70]. The impact on hematopoietic cells, particularly red blood cells, presents a substantial issue with CD47/SIRPα targeted drugs. Given that older red blood cells are more sensitive to phagocytosis, red blood cell destruction remains a limiting toxicity with these drugs, and may influence the age of patients that can be treated with these agents [
71]. Notably, as opposed to red blood cells, other normal functioning cells are less vulnerable to macrophage-mediated immune destruction with anti-CD47 therapies, as further activation of prophagocytic signals and involvement of calreticulin are suggested to be necessary steps to generate immune-related adverse events [
72,
73]. Furthermore, the use of CD47 or SIPRα-directed antibodies structured with an intact Fc portion raises similar concerns, due to the widespread expression of Fc receptors on normal cells, and the risks for potentially causing immune destruction of “self” cells [
45]. From the drug development standpoint, the efficacy of the CD47/SIRPα blockade can vary based upon drug delivery method and compartmentalization of the drug. In addition, tumor type and stage, tumor immune microenvironment and acquired drug resistance, constitute other potential determinants of the efficacy of CD47/SIRPα inhibitors [
74].
6. Anti-CD47 Antibodies and CD47-Targeting Recombinant Proteins
CD47-directed monoclonal antibodies and fusion proteins with SIRPα immunoglobulin structure competitively bind CD47 and block the interaction between CD47 and SIRPα. This class of therapeutics constitute the majority of the available in-human data testing CD47/SIRPα inhibition in solid tumors and hematologic malignancies, although data remains limited.
Hu5F9-G4 (5F9, magrolimab) is a humanized antibody with an IgG4 Fc fragment [
75]. In a preclinical setting, magrolimab demonstrated anti-tumor activity against AML in-vitro and in vivo. Furthermore, complete disease elimination was observed in human B lymphoblastoid cell-engrafted mice, after treatment with magrolimab in combination with rituximab [
75]. Preclinical models testing magrolimab in solid tumors such as colon, liver, ovarian and breast cancers demonstrated promising anti-tumor activity [
76]. Another study in which patient-derived NHL xenografted mice were treated with magrolimab/rituximab combination showed an 89% cure rate, defined as over 4 months of disease free survival following the discontinuation of therapy [
63]. In-depth analyses suggested that rituximab plays a complementary role in further stimulation of innate immunity, via its active Fc effector function-inducing natural killer cell and macrophage-mediated cellular cytotoxicity. Accordingly, the data from a phase Ib study of 22 patients with relapsed or refractory NHL, 95% of whom were previously treated with rituximab, demonstrated encouraging outcomes with an objective response rate of 50%, and a complete response rate of 36%, with magrolimab and rituximab in combination [
77]. Adverse events experienced by patients on trial included chills, anemia and headaches (41% each), all of which occurred only in the first weeks of the trial. There were no significant safety signals in the latter stages of the trial. A simultaneously conducted phase I study of single agent magrolimab in metastatic solid tumors demonstrated a similar safety profile, with transient treatment-related adverse events [
78]. Of note, trends in anemia development and transfusion requirements with magrolimab were further examined using the data from the patient population in the phase I dose escalation part of these studies [
79]. Patients on escalating doses of magrolimab experienced a median 1.0 g/dL decrease in hemoglobin levels, and subsequent doses were associated with a lesser degree of hemoglobin decline. Red blood cell transfusion yielded appropriate responses in hemoglobin concentration, supporting the evidence regarding the transient nature of anemia after magrolimab administration [
79]. A number of clinical trials evaluating magrolimab, either as a single agent or in combination with cytotoxic therapies, targeted therapies or immune checkpoint inhibitors to treat hematologic neoplasms, are ongoing.
Other CD-47-targeting monoclonal antibodies that have entered clinical development include IBI188 (letaplimab), AK117 and SRF231. A phase I study of letaplimab in patients with advanced solid tumors and lymphomas was recently completed. Letaplimab demonstrated a favorable toxicity profile, with no dose-limiting toxicities. The majority of the treatment-related adverse events were grade 1–2. The rate of anemia was 15%, and only one patient developed grade 3 anemia. Notably, infusion related reactions were seen in 65% of the patient population, but all were grade 1–2 and manageable with a standard infusion-related reaction treatment algorithm [
80]. AK117 monotherapy in patients with metastatic solid tumors demonstrated safety with no dose-limiting toxicities, no infusion-related reactions, or grade ≥ 3 treatment-related adverse events observed with up to 20 mg/kg dosing. Further dose escalation is underway with 30 mg/kg dosing [
81]. For SRF231, further exploration in clinical trials was held by the pharmaceutical company.