Antibodies are now a versatile tool for diagnostics and therapy of various conditions in humans and hyperimmune sera can be replaced by specific monoclonal antibodies (mAbs). mAbs have been known since the 1970s. Numerous mAbs have been developed against SARS-CoV 2 and have proven their effectiveness, especially in the management of the mild-to-moderate disease.
1. Introduction
Since 1901, when Emil Adolf von Behring won the Nobel Prize in Medicine for the application of animal-derived serum therapies, this approach has been attempted for several emerging infectious diseases, based on the pivotal role of the serological immune response against infectious diseases [
1]. Antibodies are now a versatile tool for diagnostics and therapy of various conditions in humans and hyperimmune sera can be replaced by specific monoclonal antibodies (mAbs). MAbs were first described almost half a century ago, deriving from mice’s vaccination with specific antigens followed by B cells harvesting from mouse spleens. Now, antibodies have become significantly easier to develop and produce whereby relevant antibodies can be identified directly from exposed persons. Investigators are now able to use flow cytometry to distinguish memory B cells based on their antigen-binding characteristics [
2]. The variable regions of the antibody heavy and light chains can be replicated, achieving monoclonal antibodies expression [
3]. From these initial procedures, candidates for mAbs development can be further selected according to in vitro neutralization assays that assess their activity. Thanks to the above mentioned progress in techniques, the necessary time to isolate and characterize antibodies has been significantly reduced.
A mAb with potential therapeutic utility should fulfill the following three conditions at least: (i) the antigen-binding fragment (Fab) domain must specifically bind to the appropriate molecular target, (ii) efficient and precise effector functions activated by binding of the constant crystallizable fragment (Fc) region to specific receptors of immune cells, and (iii) good pharmacokinetic characteristics [
1].
Initially, the application of mAbs was restricted to the development of diagnostic techniques with limited therapeutic applications, because mAbs were of animal origin exclusively, burdened by potential downsides in terms of high immunogenicity, limited half-life, and scarce capacity of activating Fc-mediated effector functions after administration. These problems were addressed by the engineering of the constant regions of an antibody molecule leading first to chimeric (murine mAbs with a human Fc fragment) and then to humanized antibodies (human mAbs, maintaining the complementarity determining regions of the original mouse mAbs) [
4]. In the last two decades, novel techniques introduced the possibility of dissecting directly the human “antibodyome,” allowing the selection of fully human mAbs [
4].
Advances in mAbs research and production could have an enormous impact in the field of medicine. Although initially devised for infectious diseases prevention or treatment, mAbs are now more extensively applied in other clinical areas as oncology or immunology.
Theoretically, mAbs could be employed against a broad variety of biologic agents, encompassing bacterial and viral pathogens, fungi, and associated toxins, with their action exerted either directly (e.g., preventing cell entry or neutralizing toxins) or via indirect mechanisms (e.g., modulating inflammatory responses or promoting opsonic phagocytosis) [
5].
Despite their potentially extensive impact, up to date, only few mAbs are licensed against infectious agents or toxins. This important field of research is constantly expanding, and new drugs are being licensed for therapeutic applications in the area of infectious diseases. For example, the FDA approved raxibacumab and obiltoxaximab, for the treatment and prophylaxis of inhalational anthrax, respectively, when alternative therapies are not available or not appropriate [
9,
10]. Moreover, ibalizumab was recently licensed as rescue therapy in heavily treatment-experienced adults with multidrug-resistant HIV-1 infection [
11].
2. Antibodies Targeting SARS-CoV-2
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread worldwide as a severe pandemic [
13]. The development of therapeutic mAbs is currently at the front line of fighting against the coronavirus disease 19 (COVID-19) pandemic and hundreds of therapeutic antibodies are either in the preclinical stages or in clinical trials [
14]. Owing to the close relatedness of SARS-CoV-2 to SARS-CoV-1, initial efforts of mAbs therapy against the former focused on repurposing anti-SARS-CoV-1 mAbs with cross-neutralizing activity against SARS-CoV-2. Later, memory B cells specific to the receptor-binding-domain (RBD) of SARS-CoV-2 spike protein were used to generate SARS-CoV-2 specific IgG1 mAbs that exhibit potent neutralizing activity [
15,
16,
17,
18].
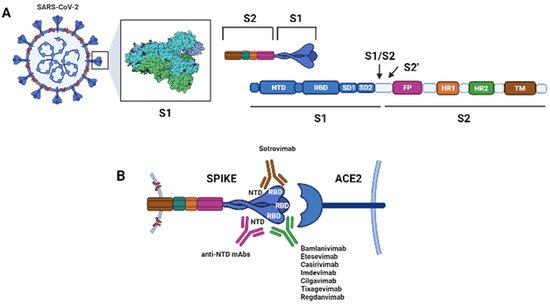
The spike (S) protein on the surface of SARS-CoV-2 binds the host cellular angiotensin-converting enzyme 2 (ACE2) receptor, allowing the virus to infect host cells. The S protein is a trimer, and each monomer comprises an N-terminal S1 subunit and a C-terminal S2 subunit. The S1 subunit further separates into the N-terminal domain (NTD), subdomain 1 (SD1), subdomain 2 (SD2) and RBD. The S2 subunit further divides into the fusion peptide (FP), the heptad repeats 1 (HR1), and heptad repeat 2 (HR2) [Figure 1]. During the virus entry phase, S1 is responsible for the receptor binding, while S2 is involved in the membrane fusion.
Figure 1. Schematic of the spike protein (S) of Sars-CoV-2 and its interactions with its cellular receptor, the angiotensin converting enzyme 2 (ACE2), and with therapeutic monoclonal antibodies (mAbs). The S protein is a trimer. (
A) Each monomer of the S protein consists of a N-terminal S1 subunit [comprising the N-terminal domain (NTD), the receptor-binding domain (RBD), subdomain 1 (SD1), and subdomain 2 (SD2)] and a C-terminal S2 subunit [comprising the fusion peptide (FP), the heptad repeat 1 (HR1), the heptad repeat 2 (HR2) and the transmembrane domain (TM)]. The S1 subunit binds the ACE2 receptor, while the S2 subunit is involved in membrane fusion during cell entry. Upon binding of the trimer to the host cell receptor through the RBD, the S1 and S2 subunits are cleaved by the host transmembrane protease serine 2 (TMPRSS2) at the S1/S2 junction; then, a second site within the S2 subunit, termed the S2′ site, is cleaved by serine proteases or cathepsins and viral-host membranes fusion is initiated. (
B) Interaction between the S protein and the host cell receptor ACE2. Most therapeutic mAb targets the RBD of the S protein at positions required for the interaction with ACE2 (Bamlanivimab, Etesevimab, Casirivimab, Imdevimab, Cilgavimab, Tixagevimab, Regdanvimab) while Sotrovimab targets the RBD, but does not compete with human ACE2 receptor binding. mAbs binding the NTD have been demonstrated to neutralize SARS-CoV-2, and these could be developed for therapeutic purposes [
15,
19,
20,
21,
22,
23,
24,
25,
26] (Created with
BioRender.com).
The S protein can be in a receptor inaccessible (closed, pre-fusion) or accessible (open, post-fusion) state according to the position of the RDB. In its open conformation, the RBD domain is exposed and able to bind ACE2; in the closed conformation, it is instead blocked.
Most neutralizing antibodies target S1, particularly the S1-RBD, due to its important function as well as its significant immunogenicity [
27,
28].
Additionally, antibodies binding the N-terminal domain of the S-protein, or a distinct proteoglycan epitope have been demonstrated to neutralize SARS-CoV-2, and could be developed for therapeutic purposes [
15,
19,
20,
21,
22,
23,
24,
25,
26]. Two mAbs developed against MERS-CoV, G2 and 7D10, target the S1-NTD region blocking the interaction between the spike protein and the host receptor DPP4. The coronavirus S2 subunit is more conserved than the S1, and carries epitopes that may represent a potential target for broadly neutralizing antibodies [
Figure 1].
The antiviral effect of mAbs is the outcome of their two functional domains: Fab that confers antigen specificity, responsible for virus neutralization, and the Fc that drives effector functions. The majority of mAbs with activity against SARS-CoV-2 bind to the S1 subunit and inhibit the virus engagement to its cell surface receptor, ACE2, with their Fab domain (neutralizing activity).
The Fc domain (constant domain) engages complement or Fc gamma receptors (FcγRs) on leukocytes promoting immune-mediated cellular clearance, the enhancement of antigen presentation and CD8+ T cell responses. Fc-FcγR interactions proved to be essential for the in vivo antiviral activity of anti-SARS-CoV-2 mAbs. MAbs lacking Fc domain showed reduced antiviral activity in vivo [
29].
Additionally, the Fc domain can reshape inflammation through engagement of FcγRs on specific cells [
30]. In particular conditions, Fc-FcγR interactions may cause antibody-dependent enhancement (ADE) of virus infection or pathological immune skewing. The effect of ADE mediated by the Fc receptor (FCR) mainly involves the interaction between the Fc fragment of the antibody and the FcγR on the cell surface. This allows the virus and antibody complex to combine with cells that express FcγR, causing the virus to adhere to their surface and increase the risk of infection.
Current evidences do not support the hypothesis that SARS-CoV-2 infection, mAbs against SARS-CoV-2 or vaccination may cause ADE effects. Although unlikely, the possibility of ADE compels a careful consideration on the role of the antibody Fc in SARS-CoV-2 therapeutic design [
31].
The neonatal Fc receptor (FcRn) is an MHC class I like molecule associated with beta-2-microglobulin (β2m) and it is involved in the IgG turnover regulating the antibody half-life in vivo mediating bidirectional transcytosis of IgG across epithelial cells as well as membrane recycling. Fc engineering has exploited the FcRn-mediated IgG recycling to develop mAbs therapeutics with improved pharmacokinetic properties, allowing lowered dosing by extending half-life and increased potency [
32].
3. Therapeutic and Prophylactic Indications
3.1. Prophylactic Use of mAb against SARS-CoV-2
Vaccines undeniably represent the most effective way to provide protection from COVID-19 for most individuals. For the last two years, the whole scientific community focused on the research, development and, ultimately, production, of a safe and effective vaccine, because it represented the only possible solution to further spreading and recurrence of SARS-CoV 2. [
52]. Vaccine development can take years, or even decades, but the aggressive efforts made to investigate several COVID-19 vaccine candidates concomitantly may significantly reduce the amount of time generally required for the development process [
53]. MAbs currently represent an alternative route of prevention for COVID-19 and could offer short-term protection to those who are not yet vaccinated or who lack a proper response to vaccination, such as immunocompromised patients. Additionally, mAbs could prove helpful during times when circulating variant viruses are not adequately covered by vaccines protection [
54].
Part A of the Regeneron trial (NCT04452318) consists of a randomized, double-blind, placebo- controlled trial to assess the efficacy and safety of the subcutaneous administration of casirivimab plus imdevimab (600/600 mg) in preventing SARS-CoV-2 infection among previously uninfected household contacts of infected persons without previous or ongoing infection. Symptomatic SARS-CoV-2 infection developed in 1.5% of participants in the casirivimab/imdevimab arm and in 7.8% in the placebo arm (relative risk reduction, 81.4%; odds ratio, 0.17;
p < 0.001) [
57].
In September 2021, and following the results of these trials, the FDA issued EUAs to allow the emergency use of bamlanivimab/etesevimab (iv) and casirivimab/imdevimab (iv or subcutaneous) for post-exposure prophylaxis of COVID-19 in individuals at high risk of progression to severe COVID-19, hospitalization or death, (patients who have not yet completed a full vaccination course or who are not expected to develop an appropriate immune response vaccination, i.e., immunocompromised individuals) who have been exposed to SARS-CoV-2 infection (close contact with an individual infected with SARS-CoV-2) or who are at high risk of exposure to an individual infected with SARS-CoV-2 because of occurrence of SARS-CoV-2 infection in other individuals in the same institutional setting (e.g., nursing homes, prisons) [
36,
38,
58].
Preliminary and interesting results in preventing SARS-CoV-2 infection in a rhesus macaque model are associated to the combination of two long-acting antibodies (cilgavimab + tixagevimab), developed from the B-cells of a convalescent donor after the infection [
40,
42]. These mAb have been engineered to have a longer half-life, to provide an expected 6–12 months of protection after a single intramuscular administration. Two clinical trials are ongoing in both pre- and post-exposure prophylaxis setting (PROVENT trial (NCT04625725); STORM CHASER trial (NCT04625972)) [
59,
60]. The PROVENT trial was conducted among participants who would supposedly benefit from prevention with long-acting antibodies due to an increased risk of inadequate response to active immunization or an increased risk of SARS-COV 2 infection, including those whose locations or circumstances put them at appreciable risk of exposure to the SARS-CoV [
60]. It is likely that this population will be the most eligible for the use of long-acting mAbs.
3.2. Use of mAb in Patient Hospitalized for COVID-19
Data supporting the use of casirivimab 4000 mg plus imdevimab 4000 mg in hospitalized patients with COVID-19 and with a negative serology for the anti-S protein antibody come from the RECOVERY study. No difference was found in 28-day all-cause mortality between standard of care and standard of care with intravenous casirivimab/imdevimab. However, in the subgroup of patients seronegative for the anti-S protein antibody, a significant reduction in 28-day all-cause mortality was found in the casirivimab plus imdevimab arm: 396 of 1633 patients (24%) died in the casirivimab plus imdevimab arm compared to 451 of 1520 patients (30%) in the standard of care arm (rate ratio 0.80; 95% CI, 0.70–0.91;
p = 0.001) [
61].
4. Challenges for Using mAbs against SARS-CoV-2
4.1. Activity against SARS-CoV-2 Variants
Viruses, including SARS-CoV-2, change over time. Most changes have a negligible impact; however, some may affect several characteristics of the virus, such as how easily it spreads, the associated disease severity, or the performance of the existing vaccines against it. Mutations in the SARS-CoV-2 spike protein could affect mAbs efficacy. Different phylogenetic nomenclatures have been used for the currently circulating SARS-CoV-2 variants. The S protein has different hotspots of mutation and deletion: those eminently involved in the immune escape process are within the RBD, such as K417N/T, N439K, L452R, Y453F, S477N, E484D/K/Q, and N501Y. It is not clear how frequently these mutations occur in mAbs-treated-patients or how they influence virus clearance.
Convergent evolution has led to different combinations of mutations among different variants. Therefore, monitoring mutations is necessary to forecast and re-adapt the inventory of therapeutic solutions [
62].
The World Health Organization (WHO) has been monitoring and assessing the evolution of SARS-CoV-2 since January 2020. Although the mutation rate of SARS-CoV-2 is lower than other RNA viruses such as the influenza virus, probably due to the viral internal proofreading mechanism, a timely detection of these mutations and their impact on treatment efficacy, or pandemic countermeasures, are very important.
The established nomenclature systems for naming and tracking SARS-CoV-2 genetic lineages by GISAID, Nextstrain, and Pango are currently and will remain in use by scientists and in scientific research. At the present time, WHO experts recommend using letters of the Greek Alphabet, i.e., Alpha, Beta, Gamma, Delta, which will be easier and more practical to be discussed by non-scientific audiences. SARS-CoV-2 variants associated with “clinical evidence” of greater transmissibility, altered virulence, or the ability to escape natural infection- and vaccine-mediated immunity or current diagnostic tests are called Variants of Concern (VoC). Other variants that posed a “potentially increased risk” to global public health are called Variants of Interest (VoI). During late 2020, the emergence of variants posing a threat to global public health prompted the characterization of specific VoCs and VoIs. See
Table 1 and
Table 2 for the currently designated VoC and VoI [
63].
Table 1. Currently designated Variants of Concern (VoC) [
63].
|
WHO Label
|
Pango
Lineage *
|
GISAID Clade
|
Nextstrain Clade
|
Additional Aamino Acid Changes
Monitored °
|
Earliest
Documented Samples
|
Date of
Designation
|
|
Alpha
|
B.1.1.7 #
|
GRY
|
20I (V1)
|
+S:484K
+S:452R
|
United Kingdom, Sep-2020
|
18 December 2020
|
|
Beta
|
B.1.351
|
GH/501Y.V2
|
20H (V2)
|
+S:L18F
|
South Africa, May-2020
|
18 December 2020
|
|
Gamma
|
P.1
|
GR/501Y.V3
|
20J (V3)
|
+S:681H
|
Brazil, Nov-2020
|
11 January 2021
|
|
Delta
|
B.1.617.2 §
|
G/478K.V1
|
21A, 21I, 21J
|
+S:417N
|
India, Oct-2020
|
VOI: 4 April 2021
VOC: 11 May 2021
|
Table 2. Currently designated Variants of Interest (VoI) [
63].
|
WHO Label §
|
Pango Lineage *
|
GISAID Clade
|
Nextstrain Clade
|
Earliest Documented Samples
|
Date of
Designation
|
|
Lambda
|
C.37
|
GR/452Q.V1
|
21G
|
Peru, December 2020
|
14 June 2021
|
|
Mu
|
B.1.621
|
GH
|
21H
|
Colombia, January 2021
|
30 August 2021
|
* includes all descendent lineages. The full list of Pango lineages can be found here: https://cov-lineages.org/lineage_list.html; for FAQ, visit: https://www.pango.network/faqs/ (accessed on 15 November 2021). § Former VOIs currently designated as VUMs: Kappa: B.1.617.1; Iota: B.1.526; Eta: B.1.525; Epsilon: B.1.427/B.1.429. Former VOIs no longer designated as VUMs: Zeta: P.2; Theta: P.3.
Monitoring resistance to mAbs among the new variants will be key to defining whether some of the newly developed mAbs should be discontinued or if different combinations should be investigated [
64].
Hereafter, we report the susceptibility of the currently designated VoC to the mAb in clinical use:
-
Alpha (B.1.1.7) variant: This VoC maintains in vitro susceptibility to all the mAbs against SARS-CoV-2 that are currently approved through EUAs [
36,
38].
-
Beta (B.1.351) variant: This VoC includes the E484K and K417N mutations, which results in a reduction in in vitro susceptibility to bamlanivimab and etesevimab [
36,
65]. In vitro studies also suggest that this variant has markedly reduced susceptibility to casirivimab, although the combination of casirivimab and imdevimab appears to retain activity; sotrovimab appears active as well against this VoC [
38,
39].
-
Gamma (P.1) variant: This VoC includes the E484K and K417T mutations, which results in a marked reduction in in vitro susceptibility to bamlanivimab and etesevimab [
36,
66]. Additionally, this variant shows reduced susceptibility to casirivimab, although the combination of casirivimab and imdevimab appears to retain activity; sotrovimab appears to retain activity as well [
38,
39].
-
Delta (B.1.617.2) variant: This is the prevalent VoC in the United States. It contains the L452R mutation, which results in a modest decrease in in vitro susceptibility to the combination of bamlanivimab and etesevimab, although the clinical implications of this finding are not fully known. Sotrovimab and casirivimab plus imdevimab appear to maintain activity [
38,
39,
67].
Mutations of SARS-CoV-2 Spike protein in VoC and resistance profile of clinical mAbs are summarized in Table 3.
Table 3. Mutations of SARS-CoV-2 S in VOC and resistance profile of clinical mAbs [
69].
| |
Casirivimab
Indevimab
|
Bamlanivimab
Etesevimab
|
Sotrovimab
|
Cilgavimab
Tixagevimab
|
Regdanvimab
|
|
B.1.1.7 (UK)
|
S
S
|
S
S
|
S
|
S
S
|
S
|
|
B.1.351 (South Africa)
|
R
S
|
R
R
|
S
|
S
S
|
I/R
|
|
P.1 (Brazil)
|
R
S
|
R
R
|
S
|
S
S
|
Pot I/R
|
|
B.1.429 (California)
|
S
S
|
R
S
|
S
|
S
S
|
I/R
|
|
B.1.1.258 (Scotland)
|
S
R
|
S
U
|
S
|
U
U
|
Pot S
|
|
B.1.525 (Nigeria)
|
Pot I/R
Pot S
|
Pot I/R
Pot S
|
S
|
Pot S
Pot S
|
U
|
|
B.1.526 (New York)
|
Pot I/R
Pot S
|
Pot I/R
Pot S
|
S
|
Pot S
Pot S
|
U
|
|
B.1.617.1 (India)
|
S
S
|
R
S
|
S
|
Pot S
Pot S
|
U
|
S = neutralized (<10-fold loss of neutralization). I/R = poorly or not-neutralized (>10-fold loss of neutralization). Pot S = potential S. Pot I/R = potential I/R. U = Unknown.
4.2. Innovative Way of mAbs Administration
Another major mAbs hurdle, in both ambulatory outpatient and prophylaxis settings, is represented by the route of administration. MAbs currently approved by the FDA for treatment of COVID-19 are administered by intravenous infusion. Subcutaneous injection is a possible alternative for the combination casirivimab plus imdevimab exclusively, when intravenous infusion is not feasible and would lead to delay in treatment [
38]. Part B of the Regeneron trial (NCT04452318) evaluated the efficacy and safety of subcutaneous casirivimab and imdevimab combination to prevent progression from early asymptomatic SARS-CoV-2 infection to COVID-19. This combination significantly prevented progression from asymptomatic to symptomatic disease compared with placebo (31.5% relative risk reduction)). The authors concluded that subcutaneous administration of this mAb prevented progression from asymptomatic SARS-CoV-2 infection to COVID-19 and was well tolerated [
57].
Specific infusion centers, pop-up sites, or in-home visits may be safer from a public health context while offering more convenient services to the patient [
54]. Several studies are focusing on mAbs administration via nasal sprays or aerosolized formulations [
70,
71,
72,
73,
74,
75,
76]. Early clinical studies have confirmed that this approach is safe and can be used to prevent and treat SARS-CoV-2 infection [
77]. The preclinical study on COVID-19-inhaled mAb demonstrated their therapeutic efficacy in animal models (mice and hamsters), significantly reducing the viral burden in SARS-CoV-2-infected animals when used either prophylactically or therapeutically [
70,
78]. Regarding the ongoing clinical trials, researchers are studying foralumab (administered by nasal spray) to treat COVID-19. Foralumab is a fully human second generation anti-CD3 mAb with a modified Fc unit composed of two heavy chains with an immunoglobulin region and two light chains with a kappa constant region.
In a Brazilian study, it was observed that in 39 patients with mild to moderate COVID-19, intranasal foralumab may be of benefit in modulating immune reactivity and in reducing pulmonary inflammation [
79]. Moreover, adeno-associated virus vectors may offer opportunities for delivering mAb-expressing gene therapy constructs [
54,
73].
This entry is adapted from the peer-reviewed paper 10.3390/ph14121272