Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Evolutionary Biology
Self-incompatibility (SI) refers to the inability of hermaphroditic angiosperms to self-pollinate, which promotes outcrossing or hybridization.
- orchids
- self-incompatibility
1. Morphological Types of SI in Orchidaceae
Many traits are used to measure the SI of orchids. Pollen tube growth, fruit set, and seed abortion rate are often used as the main indicators of SI, especially the growth of the pollen tube, as it is one of the most direct and important indicators of SI [1][2]. Although SI has been reported in various orchid groups, it is most common in a few groups, such as Dendrobiinae (i.e., Dendrobium) [3], Pleurothallidinae [4], Oncidiinae [5][6], Malaxidinae [7][8][9], Laeliinae [5][10], Aeridinae [11], Angraecinae [11], and Neottieae (i.e., Epipactis) [5], which all belong to the subfamily Epidendroideae (Figure 1). Here, we mainly review the pollen tube growth in orchid SI species.
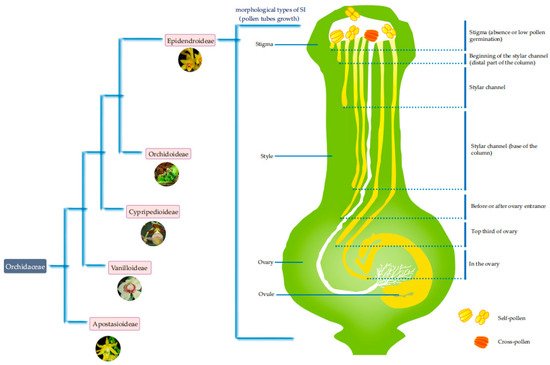
Figure 1. Diverse SI pollen tube morphologies exist in orchids in the subfamily Epidendroideae.
1.1. Subtribe Dendrobiinae
Studies of the SI of the subtribe have focused on Dendrobium. Dendrobium is the second largest genus in Orchidaceae (second only to Bulophyllum) [12], with approximately 1450 species. It is a perennial epiphytic herb that is mainly distributed in tropical, subtropical, and temperate regions of Oceania and Asia [13].
By conducting more than 1700 pollination experiments on 61 species of Dendrobium [3], Johansen found that 44/61 (72%) of the species had wilted and yellowed ovaries and showed self-sterility after self-pollination. Among the remaining 17 fruit-producing species, the fruit ripening time and seed quality varied considerably. Analysis of the development of the self-pollenated tubes of D. fameri (SI) suggested that it was consistent with the GSI phenotype given that the pollen tubes enter the style. This study indicated that some species of Dendrobium showed the GSI phenotype. Niu et al. [14] studied 26 representative species of Dendrobium and analyzed the pollen tube growth of 13 species. Four kinds of pollen tube growth phenotypes were observed in self-incompatible species: (1) pollinia did not germinate; (2) the pollen tube stopped growing at the top of the style; (3) the pollen tube stopped growing at a specific position in the stylar channel, mostly at the upper third of the stylar channel; and (4) the pollen tube stopped growing in the upper third of the ovary. For example, the pollen tube of D. densiflorum stopped growing just before or after the style entrance one day after self-pollination. The self-pollinated pollen tube of D. chrysanthum stopped growing at the upper third of the style three days after self-pollination. The pollen tube of D. lindleyi stopped growing at the point before or just after the ovary entrance [14]. The growth of the pollen tube in more than half of the self-incompatible species stops in the style, and these species are distributed in different branches of Dendrobium phylogenetic tree [14][15], which is the main SI phenotype and consistent with the GSI phenotype. The diverse pollen germination and pollen tube growth phenotypes suggest that there might be more than one molecular mechanism of SI in Dendrobium species.
All these results show significant differences to those of molecular mechanisms known in other angiosperm families. There is a surprisingly high SI phenotype diversity in orchids, even in one genus, while there is only one SI phenotype described in other angiosperm families with their molecular mechanisms known, respectively. Furthermore, the emergence times of the SI phenotypes after self-pollination varies from species to species, mostly at three to five days, but even at two to three weeks, which much longer than that (mostly from tens of minutes to hours [16][17]) in other angiosperm families with their molecular mechanisms known. Therefore, investigation of more other orchids SI species is needed, which may reveal more SI phenotypes.
1.2. Subtribe Pleurothallidinae
SI analysis of Restrepia, belonging to clade B [4] of the Pleurothallidinae, revealed that 45% of the species are self-incompatible, and seed abortion is lower for cross-pollinated plants (both intraspecific and interspecific cross-pollination) than for self-pollinated plants. Pollen tubes fill the entire ovary after three weeks of cross-pollination, but the number of self-pollinated pollen tubes decreases significantly and stops growing in the upper third of the ovary, as is the case in the R. brachypus [1]. The genus Acianthera belongs to clade C [4] of the Pleurothallidinae. After self-pollination, the pollen tube growth of A. fabiobarrosii stops in the style. Aside from the style, the ovary was found to be the second reaction site of SI, and it might be an extension of the SI reaction in the style [18].
In addition to pollen tubes, some Pleurothallidinae individuals can produce fruits with no seeds after self-pollination [4][19]. Seed abortion of A. johannensis after self-pollination may be caused by SI or by inbreeding depression [20]. What’s more, in Anathallis sclerophylla, A. heterophylla, and A. rubens, nearly all pollen grains do not germinate after self-pollination, which is a typical SSI phenotype [21], but reciprocal crosses are needed for further verification.
The characteristics of the second SI reaction site and fruits with no seeds are found in this orchid group, further surprising us. What’s more, there seems to be more SI species with self-pollenated tube growth stopping at the base of column, compared with Dendrobium species, which might suggest branch specificity. Therefore, a wider survey of the growth of pollen tube needs to be conducted, and the detailed changes in the shape of the pollen tube may also be examined, which may help answer questions on the phylogeny of SI in these groups.
1.3. Subtribe Oncidiinae
Oncidiinae has 65 genera and more than 1600 species [22][23]. Approximately 69.4% of Oncidiinae species are self-incompatible, 22.2% are self-compatible, and 8.3% have both self-incompatible and self-compatible populations [6]. The identification of SI in Oncidiinae is determined by fruit set and seed production after self-pollination [24]. In Oncidiinae, the growth of pollen tubes after self-pollination has only been reported in Notylia nemorosa. The pollen tubes either did not germinate or stopped growing at the stigmatic surface, and the flowers withered approximately four days after pollination [25].
The high SI rate in this subtribe requires pollen tube observation experiments in more species, suggesting that different SI phenotypes exist in this group and that there might be another branch-specific trait.
1.4. Subtribe Aeridinae
The SI species reported in this subtribe is Phalaenopsis pulcherrima. Zhang’s research [26] on the breeding system of P. pulcherrima has shown that there is no significant difference in fruit set between self-pollination and cross-pollination, but the number of seeds with embryos in self-pollinated plants is significantly lower compared with cross-pollinated plants. From one to four days, the extent of pollen germination is lower and the length of the pollen tube is shorter after self-pollination. On the fifth day, there are fewer pollen tubes entering the ovule after self-pollination compared with cross-pollination [26]. These findings indicate that the SI of P. pulcherrima is late-acting SI before zygote formation. In this case, pollen can germinate, and pollen tubes can grow and enter ovules; however, the large number of self-pollinated fertilized eggs cannot develop into seeds [27][28].
Based on the above listed results, the SI phenotypes in orchids species contain each developmental stage from the beginning of hydration to the formation of the zygote after self-pollination, which is not reported in other angiosperm families. However, there are still a lot of pollination experiments to be done. For orchids groups with high SI rate, pollen tube observation experiments should be carried out in more species so that more SI phenotypes may be found, further verifying whether branch specificity exists among orchids groups. For species whose pollen tube growth stops at the stigma, reciprocal cross tests need to be performed in order to distinguish between GSI and SSI. In addition, in the past, many studies have evaluated the self-incompatibility of orchids based on fruit setting rate rather than pollen tube growth, and there has been no systematic mating system study on all orchids, so the proportion of self-incompatibility in orchids has been underestimated. A wider survey of mating systems based on pollen tube growth is needed, which will help to answer questions on the phylogeny of SI among orchids groups.
2. Physiology of SI in Orchidaceae
The pollen of the Orchidaceae contains large amounts of auxins [29]. Application of auxin to the stigma can result in post-pollination phenomena [30]. The use of auxin to treat self-incompatible orchids species can trigger flower abscission, but this can lead to parthenocarpy in self-compatible or partly self-compatible plants [3][31][32]. The detection results of the pollen substances of Phalaenopsis after pollination suggest that auxin, as the primary pollen signal [33], is transferred to the style and gradually infiltrates various organs, inducing ethylene production [34] and eventually leading to apoptosis in the perianth. The concentrations of auxin and ethylene were monitored during the development of pollen tube after self- and cross-pollination in D.chrysanthum, respectively [14]. The concentration of auxin was significantly higher within three days after self-pollination compared with cross-pollination. The concentration of ethylene (ACC) increased significantly at three days after self-pollination and decreased significantly at three days after cross-pollination. SI might fine-tune the auxin concentration, which promotes the production of ethylene [14], suggesting that auxin and ethylene might be involved in SI response.
However, further experiments are needed. Investigations into external hormone and hormone inhibitor treatment would be helpful to explore the effect of hormones on pollen tube development with self-pollination. Whether auxin and ethylene are involved in the SI response in other SI phenotypic species also needs to be verified. What is more, whether other hormones and ions, such as calcium involved in the SI response in Papaveraceae [35], are also involved in the orchids’ SI response, needs further verification.
This entry is adapted from the peer-reviewed paper 10.3390/ijms222312901
References
- Millner, H.J.; McCrea, A.R.; Baldwin, T.C. An investigation of self-incompatibility within the genus Restrepia. Am. J. Bot. 2015, 102, 487–494.
- Freudenstein, J.V. Fundamentals of orchid biology. Nord. J. Bot. 1994, 14, 204.
- Johansen, B. Incompatibility in Dendrobium (Orchidaceae). Bot. J. Linn. Soc. 1990, 103, 165–196.
- Borba, E.L.; Barbosa, A.R.; Melo, M.; Gontijo, S.L.; Oliveira, H. Mating systems in the Pleurothallidinae (Orchidaceae): Evolutionary and systematic implications. Lankesteriana Int. J. Orchid. 2011, 11, 207–221.
- East, E.M. The distribution of self sterility in the flowering plants. Proc. Am. Philos. Soc. 1940, 82, 449–518.
- Castro, J.B.; Singer, R.B. A literature review of the pollination strategies and breeding systems in Oncidiinae orchids. Acta Bot. Bras. 2019, 33, 618–643.
- Oh, G.S.; Chung, M.Y.; Chung, S.G.; Chung, M.G. Contrasting breeding systems: Liparis Kumokiri and L. Makinoana (Orchidaceae). Ann. Bot. Fenn. 2001, 38, 281–284.
- Whigham, D.F.; O’Neill, J.P. Dynamics of flowering and fruit production in two eastern North American terrestrial orchids, Tipularia discolor and Liparis lilifolia. In Population Ecology of Terrestrial Orchids; Wells, T.C.E., Willems, J.H., Eds.; SPB Academic Publishers: The Hague, The Netherlands, 1991; pp. 89–101.
- Aragón, S.; Ackerman, J.D. Density effects on the reproductive success and herbivory of Malaxis massonii. Lindleyana 2001, 16, 3–12.
- Ackerman, J.D. Limitations to Sexual Reproduction in Encyclia krugii (Orchidaceae). Syst. Bot. 1989, 14, 101–109.
- Agnew, J.D. Self-Compatibility/Incompatibility in Some Orchids of the Subfamily Vandoideae. Plant Breed. 1986, 97, 183–186.
- Li, Z.-J.; Wang, Y.; Yu, Y.; Zhang, Y.; Miao, K. Studies on floral and pollination biology in endangered Dendrobium orchid. Guangdong Agric. Sci. 2009, 231, 43–49.
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. Genera Orchidacearum Volume 6: Epidendroideae; Oxford University Press: Oxford, UK, 2014.
- Niu, S.C. Morphology and Molecular Mechanism of Dendrobium Self-Incompatibility. Ph.D. Thesis, The Chinese Academy of Sciences, Beijing, China, 2018.
- Niu, S.C.; Huang, J.; Xu, Q.; Li, P.X.; Yang, H.J.; Zhang, Y.Q.; Zhang, G.Q.; Chen, L.J.; Niu, Y.X.; Luo, Y.B.; et al. Morphological type identification of self-incompatibility in Dendrobium and its phylogenetic evolution pattern. Int. J. Mol. Sci. 2018, 19, 2595.
- Dickinson, H. Dry stigmas, water and self-incompatibility in Brassica. Sex. Plant Reprod. 1995, 8, 1–10.
- Elleman, C.J.; Franklin-Tong, V.; Dickinson, H.G. Pollination in species with dry stigmas—The nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol. 1992, 121, 413–424.
- Duarte, M.O.; Oliveira, D.; Borba, E.L. Two self-incompatibility sites occur simultaneously in the same Acianthera species (Orchidaceae, Pleurothallidinae). Plants 2020, 9, 1758.
- Borba, E.L.; Semir, J.; Shepherd, G.J. Self-incompatibility inbreeding depression and crossing potential in five Brazilian Pleurothallis (Orchidaceae) Species. Ann. Bot. 2001, 88, 89–99.
- Duarte, M.O. Sementes Abortadas ou Óvulos não Fecundados? Investigação da Possível Ocorrência de Dois Sítios de Autoincompatibilidade em Acianthera (Orchidaceae). Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 2020.
- Gontijo, S.L.; Barbosa, A.R.; de Melo, M.C. Occurrence of different sites of self-incompatibility reaction infour Anathallis (Orchidaceae, Pleurothallidinae) species. Plant Spec. Biol. 2010, 25, 129–135.
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174.
- Neubig, K.M.; Whitten, W.M.; Williams, N.H.; Blanco, M.A.; Endara, L.; Burleigh, J.G.; Silvera, K.; Cushman, J.C.; Chase, M.W. Generic recircumscriptions of Oncidiinae (Orchidaceae: Cymbidieae) based on maximum likelihood analysis of combined DNA datasets. Bot. J. Linn. Soc. 2012, 168, 117–146.
- Tremblay, R.L.; Ackerman, J.D.; Zimmerman, J.K.; Calvo, R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol. J. Linn. Soc. 2005, 84, 1–54.
- Singer, R.B.; Koehler, S. Notes on the Pollination Biology of Notylia nemorosa (Orchidaceae): Do pollinators necessarily promote cross pollination? J. Plant Res. 2003, 116, 19–25.
- Zhang, Z. Conservation Biology of Three Phalaenopsis (Orchidaceae) Species in Hainan Island. Ph.D. Thesis, Hainan University, Haikou, China, 2018.
- De Nettancourt, D. Incompatibility and Incongruity in Wild and Cultivated Plants; Springer: New York, NY, USA, 2001.
- Chai, L.; Ge, X.; Biswas, M.K.; Qiang, X.; Deng, X. Self-sterility in the Mutant ‘zigui Shatian’ Pummelo (Citrus grandis Osbeck) Is Due to Abnormal Post-zygotic Embryo Development and Not Self-incompatibility. Plant Cell Tissue Organ Cult. 2011, 104, 1–11.
- Müller, R. Zur quantitativen Bestimmung von Indolylessigsaure mittels Papirchromatographie und Papirelektrophorese. Beitr. Biol. Pflanz. 1953, 30, 1–32.
- Arditti, J.; Flick, B.H. Post-pollination Phenomena in Orchid Flowers. Vi. Excised Floral Segments of Cymbidium. Am. J. Bot. 1976, 63, 201–211.
- Heslop-Harrison, J. The physiology of reproduction in Dactylorchis. I. Auxin and the control of meiosis, ovule formation and ovary growth. Rotaniska Not. 1957, 110, 28–48.
- Gregory, L.E.; Gaskins, M.H.; Colberg, C. Parthenocarpic Pod Development By Vanilla planifolia Andrews Induced with Growth-regulating Chemicals. Econ. Bot. 1967, 21, 351–357.
- Zhang, X.S.; O’Neill, S.D. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell 1993, 5, 403–418.
- Burg, S.P.; Dijkman, M.J. Ethylene and Auxin Participation in Pollen Induced Fading of Vanda Orchid Blossoms. Plant Physiol. 1967, 42, 1648–1650.
- Fujii, S.; Kubo, K.; Takayama, S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants 2016, 2, 16130.
This entry is offline, you can click here to edit this entry!
