Glucocorticoids, as multifunctional hormones, are widely used in the treatment of various diseases including nephrological disorders. They are known to affect immunological cells, effectively treating many autoimmune and inflammatory processes. Furthermore, there is a growing body of evidence demonstrating the potent role of glucocorticoids in non-immune cells such as podocytes. Moreover, novel data show additional pathways and processes affected by glucocorticoids, such as the Wnt pathway or autophagy. The endothelium is currently considered as a key organ in the regulation of numerous kidney functions such as glomerular filtration, vascular tone and the regulation of inflammation and coagulation.
1. Introduction
Glucocorticoids (GCs) are multifunctional hormones affecting the human metabolism, the immunological system, reproduction, circadian rhythm and several other vital functions. This implies the importance of GCs in the treatment of a wide spectrum of diseases such as autoimmune and inflammatory processes and malignancies [
1,
2]. For many years, they have been a staple in the treatment of a variety of kidney diseases such as glomerulonephritis [
3]. Despite this success, they can cause several side effects such as infections or compromised metabolism [
4]. In some cases, treatment resistance might occur [
4]. Those factors increase interest in GC function and signalling in different cells and tissues to minimise side effects and increase the efficacy of GC curation. On the other hand, novel studies have unravelled the roles of GCs and their receptors in the pathology of different diseases and processes [
4]. Furthermore, GCs play a variety of roles, sometimes even opposing ones, in different tissues and organs [
5]. Research in recent years brought evidence of multifactorial effect of sex hormones and mineralocorticoids on kidney function and structure, especially in diabetic kidney disease [
6,
7]. Moreover, other studies revealed a wide-array of functions of nuclear receptors in podocytes [
8]. One novel study drew attention to the endothelial glucocorticoid receptor function in diabetic kidney disease [
9]. In this review, we will focus on the role of the endothelial glucocorticoid receptor (GR) in the pathogenesis of kidney diseases (
Table 1).
Table 1. Patomechanism and potential role of endothelial glucocorticoid receptor in the pathogenesis of particular diseases.
| Endothelial Glucocorticoid Receptor in the Pathogenesis of Particular Diseases |
Pathomechanism |
| Hypertension and vascular diseases |
-
GCs increase AT1 receptor concentration and change the flow of Na+ and Ca2+
-
GCs can increase the secretion of ET-1 and AT-2 from ECs causing vascular smooth muscle contraction
-
GCs directly reduce eNOS transcription
|
| Cardio-vascular diseases |
|
| Diabetic nephropathy |
|
| Chronic kidney disease |
|
In humans, GCs are produced mainly by the adrenal glands under the regulation of the hypothalamic–pituitary–adrenal axis and their secretion depends on various factors such as stress or daily rhythm [
2]. There is also evidence of local production of GCs in other organs, mainly immunocompetent organs such as the skin, thymus or intestines, although their function is still uncertain [
10].
2. Role of Endothelial Glucocorticoid Receptor in the Pathogenesis of Arterial Hypertension and Vascular Diseases
Hypertension is a common feature of Cushing syndrome which also affects nearly 20% of patients treated with GCs. The pathogenesis of this event is complex and still uncertain, affecting GC signalling in different tissues. Traditionally, it has been associated with mineralocorticoid function and sodium conservation, taking place mainly in the distal nephron, but novel data suggest that many other mechanisms might be involved in this process. Numerous studies, chiefly in animals, have pointed out the important role of vascular contractility affected by both endothelium and smooth muscle cells. In smooth muscles, GCs increase AT1 receptor concentration and change the flow of Na
+ and Ca
2+. In the central nervous system, GCs disturb neuronal NO release [
31]. Salt-sensitive hypertension occurring in individuals with reduced kidney mass, both congenital and for example after unilateral nephrectomy, might be associated more with the function of GR than MR. A controlled study on rats fed a high-salt diet after unilateral nephrectomy showed that hypertension can be reversed by an MR antagonist but not an aldosterone synthase inhibitor. A significant disbalance of 11β-hydroxysteroid dehydrogenases, favouring the production of active GR in kidney tissue, has also been found. This can be explained by the fact that GCs have similar affinity for both GR and MR and their action in kidney is controlled by 11β-hydroxysteroid dehydrogenase 2 and local inactivation [
32]. In so-called aldosterone-sensitive distal nephron, this enzymatic deactivation protects kidney epithelial cells from GC-mediated activation and allows for more precise regulation by mineralocorticoids [
14]. A study on MR knockout mice demonstrated that expression of the epithelial Na
+ channel and improvement of mineral balance can be mediated solely by GC therapy [
33]. Interestingly, research on distal nephron GR knockout mice demonstrated that its function is not required for the development and maintenance of GC-induced hypertension [
34]. Furthermore, another study on mice with local, tubular GR knockout showed only a transient effect on sodium handling despite the reduction in the sodium chloride cotransporter (NCC) expression [
35]. On the other hand, GCs downregulate and inhibit vasopressin receptor, V2R, in the rat inner medullary collecting duct, causing water and sodium secretion after water overload [
36].
GCs can cause various effects in ECs associated with blood pressure regulation. Primarily, they can increase the secretion of ET-1 and AT-2 from ECs, causing vascular smooth muscle contraction [
26]. Secondarily, they affect NO action in many stages. GCs directly reduce eNOS transcription through GATA interaction in human cell lines. Furthermore, they increase eNOS mRNA degradation and reduce protein stability. In addition, GCs can lower intracellular Ca
2+ mobilisation and tamper with the reaction of cells to such stimulants as ATP. The next potential mechanism reducing NO synthesis could be the inhibition of GTP cyclohydrolase—the enzyme responsible for the production of tetrahydrobiopterin, an eNOS cofactor. In animals and in cell cultures, GCs reduce prostacyclin production via phospholipase A2 and potentially COX-1 inhibition (in foetal cells) [
37]. A study on mice demonstrated that endothelial GR knockout animals do not develop hypertension after dexamethasone administration. Although those mice had a higher baseline blood pressure (BP) than matched controls, their BP did not change significantly on administration of early or chronic oral dexamethasone. Endothelial GR knockout animals did not present elevated natriuresis in contrast to the control group. Both groups showed a similar decrease in NO production after dexamethasone treatment, but GR knockout mice had a decreased arteriole contractile response to the drug. Interestingly, vascular reactivity to phenylephrine was similar in both groups. In the case of circadian BP rhythm, while it changed significantly in both groups, leading to higher BP in resting hours, it partly returned to normal after 2–7 days in endothelial GR knockout mice [
38]. A different study demonstrated that dexamethasone administration increases ROS production in HUVECs as another factor interfering with NO production [
39].
In an animal sepsis model, endothelial GR plays a protective role by mitigating iNOS and eNOS activation and might be seen as a negative regulator of eNOS. Endothelial GR knockout mice had a higher mortality rate after LPS injection due to cardiovascular shock. Moreover, studies on HUVECs have shown that GR knockdown by siRNA augments the inflammatory response by upregulation of the NF-κB pathway [
40]. This pathological effect could not be reversed by previous dexamethasone administration. In fact, endothelial GR knockout mice had worse prognosis with additional dexamethasone treatment. These studies indicate the importance of endothelial GR in the pathogenesis of septic shock and BP regulation in animal models [
41]. On the other hand, previous studies have demonstrated that dexamethasone can activate eNOS in a non-genomic manner in a mouse model of ischaemia–reperfusion injury. In this model, GCs showed a protective role in reducing vascular inflammation and reducing the myocardial infarct area [
42].
At the moment, it is assumed that atherosclerosis originates due to two events: EC damage and vascular remodelling. The consequences are increased endothelial permeability and recruitment of circulating inflammatory cells through ICAM-1 and VCAM-1 expression. This allows the migration of LDL into the vascular wall and further inflammation. Traditionally, an excess of GCs, as observed in Cushing syndrome, is connected with higher cardiovascular risk and development of atherosclerosis but novel studies of both animal models and cell lines bring conflicting results. Despite the known role of GCs in the downregulation of EC adhesion molecules and inhibition of IL-6, IL-8 and CCL which should be beneficial, some animal models show pro-atherogenic effects [
26].
Endothelial GR is important in the pathophysiology of atherosclerosis. Studies using endothelial GR knockout mice fed with a high-fat diet showed greater atherosclerosis in comparison to wild genotype mice. It is postulated that the action of endothelial GR attenuates atherosclerosis and vascular inflammation [
43]. Due to the multifactorial pathophysiology of atherosclerosis, the effect might be dependent on the different cells involved and other aspects [
26].
3. Role of Endothelial Glucocorticoid Receptor in the Pathogenesis of Glomerulopathies
For many years, GCs have been used as a cornerstone in the therapy of most types of glomerulonephritis, both primary and secondary, and in some, such as minimal change disease, they can be successfully used in monotherapy. In others, such as crescentic glomerulonephritis in vasculitis or severe cases of lupus nephritis, they fail to establish sustainable remission without the addition of other immunosuppressive drugs [
3]. These heterogeneous clinical results have generated interest in the precise action of GCs in glomeruli. The therapeutic effect of GCs had been traditionally attributed to the mitigation of inflammation directly in immune cells. Novel studies unravel a more complex relationship. For example, GCs protect podocytes through stabilisation of the slit diaphragm and cytoskeleton and preserve podocyte differentiation after injury [
44]. Moreover, mice with GR knockout in podocytes are more vulnerable to kidney injury by various factors and show worse proteinuria and podocyte foot process effacement. This has been attributed to defective cytoskeleton formation and migration after podocyte injury [
45]. Interestingly, some studies have revealed a potential negative effect of GR stimulation. One study analysed GR inactivation (selective GR knockout or GR antagonist treatment) in kidney epithelial cells in a mouse model of crescentic glomerulonephritis. It led to a beneficial effect, reduced albuminuria and reduced crescent formation parallel to GC treatment results, but without any major side effects. This was achieved chiefly by inhibition of peripheral epithelial cell proliferation and activating a process associated with crescent formation [
46]. The role of endothelial GR in glomerulopathies is less elucidated (
Table 2). We already know that normal structure and function of endothelium is crucial for glomerular filtration and protection against thrombosis and inflammation. Based on the data from animals, cell lines and human tissue samples, we know that NF-κB signalling in ECs mediated by ANCA-stimulated neutrophils is a key pathogenetic factor causing ANCA-associated vasculitis [
47]. Another important process responsible for appropriate glomerular filtration barrier function is podocyte–endothelium crosstalk (
Figure 2). One of the major factors involved in this action is VEGF [
48]. Mice overexpressing VEGFR in podocytes develop a nephrotic syndrome similar to minimal change disease observed in humans. It is mediated chiefly by podocyte foot process effacement without significant endothelial damage [
49]. It has been previously demonstrated that plasma and urinary VEGF levels are elevated during the onset of nephrotic syndrome in humans and decline after GC treatment [
50]. On the other hand, VEGF depletion can cause endothelial injury and proteinuria in a process called endotheliosis. It is assumed as a main pathophysiological lesion in pre-eclampsia or anti-VEGF cancer treatment complications [
48,
51,
52].
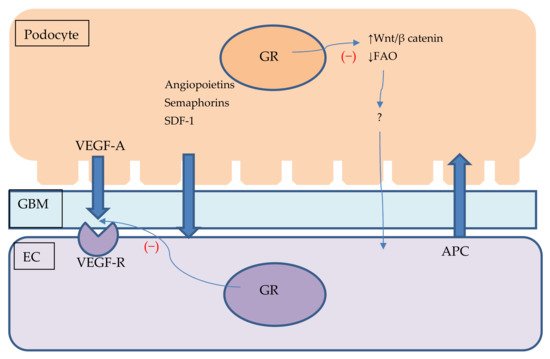
Figure 2. Podocyte–endothelial crosstalk and its potential connection with GR signalling. Figure shows podocyte and endothelial cell with glomerular base membrane between them. Podocytes produce VEGF, angiopoietins, semaphorins and SDF-1 which are secreted and influence endothelial cells homeostasis. ECs might secrete APC which protects podocytes against apoptosis. GR stimulation might impair VEGF signalling and influence podocyte Wnt/β catenin pathway and promote FAO. Lack of GR stimulation in podocytes might damage ECs through unknown mediator. APC—activated protein C, EC—endothelial cell, FAO—fatty acid oxidation, GBM—glomerular base membrane, SDF-1—Stromal cell-derived factor 1 VEGF—vascular endothelial growth factor, VEGF-R—vascular endothelial growth factor receptor, Wnt—Wnt signalling pathway.
Table 2. Potential links between glomerulopathies and abnormal GR signalling.
| Glomerulonephritis |
Pathogenesis of Selected Glomerulonephritis |
| Focal segmental glomerulosclerosis (FSGS) |
|
| ANCA-associated vasculitis |
|
| Minimal change disease |
|
| Lupus nephritis |
|
Glomerular ECs compared with other ECs produce more fibrinolytic factors and have a different metabolic profile, leading to their vulnerability to oxidative stress and membrane lipid peroxidation [
53]. Damage to ECs is primarily responsible for the pathogenesis of lupus nephritis, vasculitis, TMA and antibody-mediated rejection of kidney transplant [
18,
54,
55].
Previous studies have demonstrated a potent anti-apoptotic effect of GCs in cultured bovine ECs. Exposure of cells to TNF-α or LPS causes apoptosis and this effect is inhibited by the addition of dexamethasone. There is a time window up to 18 h after cell insult when dexamethasone application shows a protective outcome. The nature of this reaction is still unclear. It has been clearly attributed to GR action and processes upstream of caspase activation, mainly changes in the composition of bcl-2-related proteins. On the other hand, the anti-apoptotic effect could be only partly linked with the early stages of apoptosis activation and mitochondrial permeability transition. Similar results of an anti-apoptotic effect of dexamethasone have been observed in an EC line exposed to fluvastatin [
56,
57,
58].
Some types of glomerulonephritis, for example, those associated with vasculitis, cannot be treated effectively with GC monotherapy. An insight into this phenomenon has been given by analysing the effect of GCs in different cells. A study on cell cultures comparing the reaction to GCs in monocytes and ECs in standard and inflammatory milieus showed a contrasting result. In monocytes, GCs suppressed pro-inflammatory genes such as TNFSF10, IL1B and CCL5 and induced anti-inflammatory genes such as IL1R2, DUSP1, FPR1 and FKBP5. Interestingly, GCs failed to present this effect in HUVEC cultures. Further analysis and gene profiling revealed that while GCs suppressed 22% to 28% of immune response-associated genes in monocytes, they were able to suppress only 2% of those genes in HUVECs. This difference in GC action could not be explained by different expression of GR or its impaired translocation but might be associated with decreased induction of SAP30—a subunit of the Sin3A–HDAC corepressor complex [
59]. Under other conditions, previous studies have shown that GCs can decrease activation of the MAPK signalling pathway, providing an anti-inflammatory effect in human microvascular ECs acquired from lungs. These results have not been tested in ECs acquired from kidneys [
60]. In a different study, GCs inhibited IL-6 production but failed to interact with VCAM-1 induction in HUVECs under inflammatory conditions. This could be explained by the different coactivators necessary for GR–NF-κB interaction with IL-6 and VCAM-1 promotors [
61].
It is also important to note that other nuclear receptors such as vitamin D receptor, retinoic acid receptor α and oestrogen receptors play important role in podocyte function and they interaction with GR in case of podocyte injury and homoeostasis might have further clinical implications [
8,
62].
This entry is adapted from the peer-reviewed paper 10.3390/ijms222413295