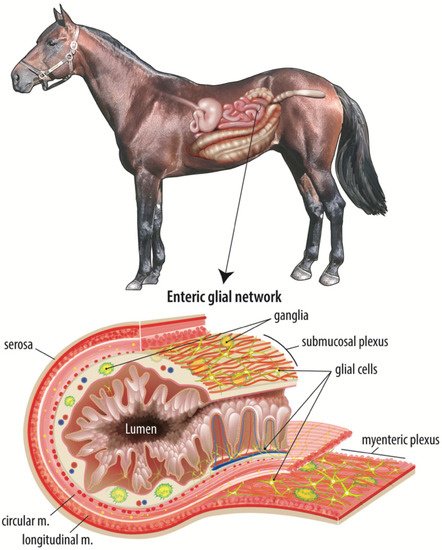
Colic is a leading cause of death in horses, with the most fatal form being strangulating obstruction which directly damages the intestinal barrier. Following surgical intervention, it is imperative that the intestinal barrier rapidly repairs to prevent translocation of gut bacteria and their products and ensure survival of the patient. Age-related disparities in survival have been noted in many species, including horses, humans, and pigs, with younger patients suffering poorer clinical outcomes. Maintenance and repair of the intestinal barrier is regulated by a complex mucosal microenvironment, of which the ENS, and particularly a developing network of subepithelial enteric glial cells, may be of particular importance in neonates with colic. Postnatal development of an immature enteric glial cell network is thought to be driven by the microbial colonization of the gut and therefore modulated by diet-influenced changes in bacterial populations early in life.
- horse
- colic
- ischemia-reperfusion injury
- enteric nervous system
- intestinal barrier
1. Evidence of Age-Dependent Barrier Repair: A Comparative Pig Model
2. A Complex Mucosal Microenvironment Regulates Epithelial Repair
This entry is adapted from the peer-reviewed paper 10.3390/ani11123337
References
- Pohl, C.S.; Medland, J.E.; Moeser, A.J. Early-life stress origins of gastrointestinal disease: Animal models, intestinal pathophysiology, and translational implications. Am. J. Physiol. Liver Physiol. 2015, 309, G927–G941.
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.F.; Blisklager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Liver Physiol. 2010, 298, G352–G363.
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 716–724.
- Blikslager, A.T.; Roberts, M.C.; Gerard, M.P.; A Argenzio, R. How important is intestinal reperfusion injury in horses? J. Am. Veter. Med. Assoc. 1997, 211, 1387–1389.
- Blikslager, A.T.; Roberts, M.C.; Rhoads, J.; A Argenzio, R. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery 1997, 121, 526–534.
- Granger, D.N.; Holm, L.; Kvietys, P. The Gastrointestinal Circulation: Physiology and Pathophysiology. Compr. Physiol. 2015, 5, 1541–1583.
- Bellamy, J.E.C.; Nielsen, N.O.; Latshaw, W.K. The Vascular Architecture of the Porcine Small Intestine. Can. J. Comp. Med. Rev. Can. Med. Comp. 1973, 37, 56–62.
- Pohl, C.S.; Medland, J.E.; Mackey, E.; Edwards, L.L.; Bagley, K.D.; Dewilde, M.P.; Williams, K.J.; Moeser, A.J. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 2017, 29, e13118.
- Medland, J.E.; Pohl, C.S.; Edwards, L.L.; Frandsen, S.; Bagley, K.; Li, Y.; Moeser, A.J. Early life adversity in piglets induces long-term upregulation of the enteric cholinergic nervous system and heightened, sex-specific secretomotor neuron responses. Neurogastroenterol. Motil. 2016, 28, 1317–1329.
- Ziegler, A.L.; Pridgen, T.A.; Mills, J.K.; Gonzalez, L.M.; Van Landeghem, L.; Odle, J.; Blikslager, A.T. Epithelial restitution defect in neonatal jejunum is rescued by juvenile mucosal homogenate in a pig model of intestinal ischemic injury and repair. PLoS ONE 2018, 13, e0200674.
- McCann, C.; Alves, M.M.; Brosens, E.; Natarajan, D.; Perin, S.; Chapman, C.; Hofstra, R.M.; Burns, A.J.; Thapar, N. Neuronal Development and Onset of Electrical Activity in the Human Enteric Nervous System. Gastroenterology 2019, 156, 1483–1495.e6.
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294.
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979.
- Gong, W.; Guo, M.; Han, Z.; Wang, Y.; Yang, P.; Xu, C.; Wang, Q.; Du, L.; Li, Q.; Zhao, H.; et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016, 7, e2387.
- Sémont, A.; Mouiseddine, M.; François, A.; Demarquay, C.; Mathieu, N.; Chapel, A.; Saché, A.; Thierry, D.; Laloi, P.; Gourmelon, P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2009, 17, 952–961.
- Ocansey, D.W.; Wang, L.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Mesenchymal stem cell–gut microbiota interaction in the repair of inflammatory bowel disease: An enhanced therapeutic effect. Clin. Transl. Med. 2019, 8, 31.
- Swerlick, R.A.; Lawley, T.J. Role of Microvascular Endothelial Cells in Inflammation. J. Investig. Dermatol. 1993, 100, S111–S115.
- Saps, M.; Miranda, A. Gastrointestinal Pharmacology. Mediat. Drugs Gastrointest. Motil. I 2017, 239, 147–176.
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, S.T.N.S.J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722.
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12.
- Qin, X.; Caputo, F.J.; Xu, D.-Z.; Deitch, E.A. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock 2008, 29, 372–376.
- Rupani, B.; Caputo, F.J.; Watkins, A.; Vega, D.; Magnotti, L.J.; Lu, Q.; Xu, D.Z.; Deitch, E.A. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery 2007, 141, 481–489.
- Turner, J.R.; Rill, B.K.; Carlson, S.L.; Carnes, D.; Kerner, R.; Mrsny, R.J.; Madara, J.L. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am. J. Physiol. Content 1997, 273, C1378–C1385.
