The biochar biofilter proved to be 96% efficient at cleaning HCH and its transformation products from drainage water, a significant improvement over classic biofilter that remove, on average, 68% of HCH. Although iron- and sulfur-oxidizing bacteria, such as Gallionella and Sulfuricurvum, were dominant in the biochar bed outflows, they were absent in sediments, which were rich in Simplicispira, Rhodoluna, Rhodoferax, and Flavobacterium. The presence of functional genes involved in the biodegradation of HCH isomers and their byproducts was confirmed in both systems. The high effectiveness of the biochar biofilter displayed in this study should further encourage the use of biochar in water treatment solutions, e.g., for temporary water purification installations during the construction of other long-term wastewater treatment technologies, or even as final solutions at contaminated sites.
- biodegradation
- biochar
- constructed wetlands
- hexachlorocyclohexane
- microbial communities
- biofilter
- lindane
1. Introduction
2. General Characteristics of Inflow Water
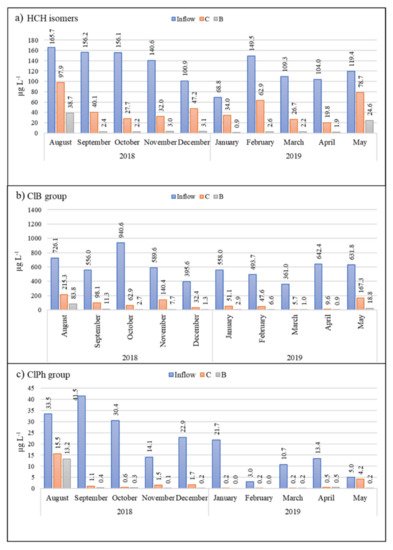
3. Pollutant Removal Efficiency
3.1. HCH Isomers
3.2. Chlorobenzenes
-
From August to November, the decrease was oscillatory (58.2%, 28%, 57.4%, and 41%, respectively);
-
From December (8.3%), its removal consistently increased and in April reached 84.4%.
3.3. Chlorophenols
4. Microbial Community Structure and Function
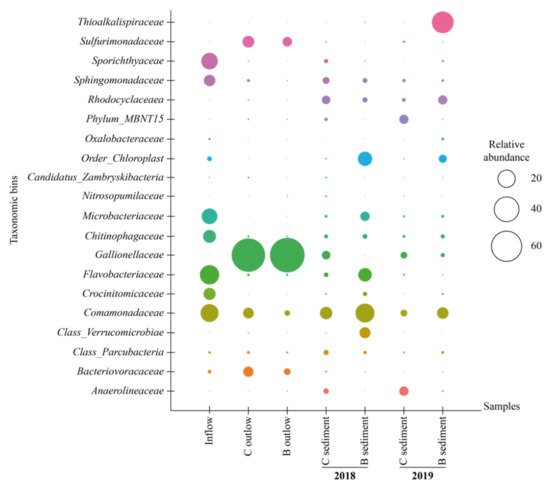
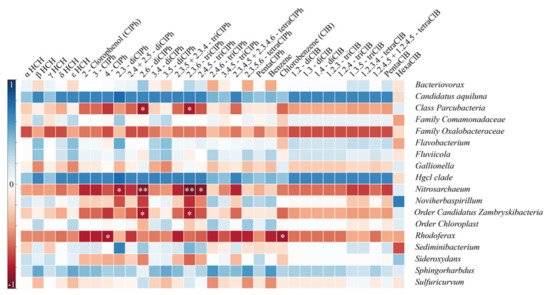
5. Functional Genes Involved in HCH Biodegradation
| Gene | ||||||
|---|---|---|---|---|---|---|
| Biofilter | U16SRT | linA | linB | linB-RT | linD | |
| 2018 | C | +++ | +++ | ++ | +++ | +++ |
| B | + | ND | ND | ND | ND | |
| 2019 | C | +++ | ++ | ++ | ++ | ++ |
| B | +++ | +++ | +++ | +++ | +++ | |
This entry is adapted from the peer-reviewed paper 10.3390/w13233396
References
- Stockholm Convention—An Overview ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/stockholm-convention (accessed on 21 June 2021).
- Bajpai, A.; Shukla, P.; Dixit, B.S.; Banerji, R. Concentrations of organochlorine insecticides in edible oils from different regions of India. Chemosphere 2007, 67, 1403–1407.
- Chakraborty, P.; Zhang, G.; Li, J.; Sampathkumar, P.; Balasubramanian, T.; Kathiresan, K.; Shin Takahashi, S.; Subramanian, A.; Tanabe, S.; Jones, C.K. Seasonal variation of atmospheric organochlorine pesticides and polybrominated diphenyl ethers in Parangipettai, Tamil Nadu, India: Implication for atmospheric transport. Sci. Total Environ. 2019, 649, 1653–1660.
- Erkmen, B.; Kolankaya, D. Determination of organochlorine pesticide residues in water, sediment, and fish samples from the Meriç Delta, Turkey. Int. J. Environ. Anal. Chem. 2006, 86, 161–169.
- Rigét, F.; Bignert, A.; Braune, B.; Dam, M.; Dietz, R.; Evans, M.; Green, N.; Gunnlaugsdottir, H.; Hoydal, K.S.; Kucklick, J.; et al. Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota. Sci. Total Environ. 2019, 649, 99–110.
- Zhao, G.; Xu, Y.; Li, W.; Han, G.; Ling, B. PCBs and OCPs in human milk and selected foods from Luqiao and Pingqiao in Zhejiang, China. Sci. Total Environ. 2007, 378, 281–292.
- ERA-Consult Madrid, Directorate-General for Internal Policies of the Union (European Parliament), M. Vega, D. Romano, and E. Uotila. Lindane (Persistant Organic Pollutant) in the EU. 30 January 2017. Available online: http://op.europa.eu/en/publication-detail/-/publication/8e37ab69-bd13-11e6-a237-01aa75ed71a1 (accessed on 24 May 2021).
- Breivik, K.; Pacyna, J.M.; Münch, J. Use of α-, β- and γ-hexachlorocyclohexane in Europe, 1970–1996. Sci. Total Environ. 1999, 239, 151–163.
- Dominguez, C.M.; Parchão, J.; Rodriguez, S.; Lorenzo, D.; Romero, A.; Santos, A. Kinetics of Lindane Dechlorination by Zerovalent Iron Microparticles: Effect of Different Salts and Stability Study. Ind. Eng. Chem. Res. 2016, 55, 12776–12785.
- Homolková, M.; Hrabák, P.; Kolář, M.; Černík, M. Degradability of hexachlorocyclohexanes in water using ferrate (VI). Water Sci. Technol. 2015, 71, 405–411.
- Joo, S.H.; Zhao, D. Destruction of lindane and atrazine using stabilized iron nanoparticles under aerobic and anaerobic conditions: Effects of catalyst and stabilizer. Chemosphere 2008, 70, 418–425.
- Merz, J.P.; Gamoke, B.C.; Foley, M.P.; Raghavachari, K.; Peters, D.G. Electrochemical reduction of (1R,2r,3S,4R,5r,6S)-hexachlorocyclohexane (Lindane) at carbon cathodes in dimethylformamide. J. Electroanal. Chem. 2011, 660, 121–126.
- Wacławek, S.; Silvestri, D.; Hrabák, P.; Padil, V.V.; Torres-Mendieta, R.; Wacławek, M.; Černík, M.; Dionysiou, D. Chemical oxidation and reduction of hexachlorocyclohexanes: A review. Water Res. 2019, 162, 302–319.
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282.
- Denyes, M.J.; Langlois, V.S.; Rutter, A.; Zeeb, B.A. The use of biochar to reduce soil PCB bioavailability to Cucurbita pepo and Eisenia fetida. Sci. Total Environ. 2012, 437, 76–82.
- Paul, A.; Ghosh, C.; Boettinger, W.J. Diffusion Parameters and Growth Mechanism of Phases in the Cu-Sn System. Metall. Mater. Trans. A 2011, 42, 952–963.
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202.
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622.
- Silvani, L.; Cornelissen, G.; Hale, S.E. Sorption of α-, β-, γ- and δ-hexachlorocyclohexane isomers to three widely different biochars: Sorption mechanisms and application. Chemosphere 2019, 219, 1044–1051.
- Xu, X.; Shi, Z.; Li, D.; Rey, A.; Ruan, H.; Craine, J.M.; Liang, J.; Zhou, J.; Luo, Y. Soil properties control decomposition of soil organic carbon: Results from data-assimilation analysis. Geoderma 2016, 262, 235–242.
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179.
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183.
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33.
- Grgić, M.; Maletić, S.; Beljin, J.; Isakovski, M.K.; Rončević, S.; Tubić, A.; Agbaba, J. Lindane and hexachlorobenzene sequestration and detoxification in contaminated sediment amended with carbon-rich sorbents. Chemosphere 2019, 220, 1033–1040. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201900062586 (accessed on 1 May 2020).
- Lal, R.; Pandey, G.; Sharma, P.; Kumari, K.; Malhotra, S.; Pandey, R.; Raina, V.; Kohler, H.-P.E.; Holliger, C.; Jackson, C.; et al. Biochemistry of Microbial Degradation of Hexachlorocyclohexane and Prospects for Bioremediation. Microbiol. Mol. Biol. Rev. MMBR 2010, 74, 58–80.
- Bala, K.; Sharma, P.; Lal, R. Sphingobium quisquiliarum sp. nov., a hexachlorocyclohexane (HCH)-degrading bacterium isolated from an HCH-contaminated soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 429–433.
- Böltner, D.; Moreno-Morillas, S.; Ramos, J.-L. 16S rDNA phylogeny and distribution of lin genes in novel hexachlorocyclohexane-degrading Sphingomonas strains. Environ. Microbiol. 2005, 7, 1329–1338.
- Mohn, W.W.; Mertens, B.; Neufeld, J.D.; Verstraete, W.; Lorenzo, V.D. Distribution and phylogeny of hexachlorocyclohexane-degrading bacteria in soils from Spain. Environ. Microbiol. 2006, 8, 60–68.
- Saxena, A.; Anand, S.; Dua, A.; Sangwan, N.; Khan, F.; Lal, R. Novosphingobium lindaniclasticum sp. nov., a hexachlorocyclohexane (HCH)-degrading bacterium isolated from an HCH dumpsite. Int. J. Syst. Evol. Microbiol. 2013, 63, 2160–2167.
- Nagata, Y.; Miyauchi, K.; Takagi, M. Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 1999, 23, 380–390.
- Oakley, A.J.; Klvaňa, M.; Otyepka, M.; Nagata, Y.; Wilce, M.C.J.; Damborský, J. Crystal Structure of Haloalkane Dehalogenase LinB from Sphingomonas paucimobilis UT26 at 0.95 Å Resolution: Dynamics of Catalytic Residues. Biochemistry 2004, 43, 870–878.
- Okai, M.; Kubota, K.; Fukuda, M.; Nagata, Y.; Nagata, K.; Tanokura, M. Crystal Structure of γ-Hexachlorocyclohexane Dehydrochlorinase LinA from Sphingobium japonicum UT26. J. Mol. Biol. 2010, 403, 260–269.
- Brittain, D.R.B.; Pandey, R.; Kumari, K.; Sharma, P.; Pandey, G.; Lal, R.; Coote, M.L.; Oakeshott, J.G.; Jackson, C.J. Competing SN2 and E2 reaction pathways for hexachlorocyclohexane degradation in the gas phase, solution and enzymes. Chem. Commun. 2010, 47, 976–978.
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593.
- Cui, L.; Yin, C.; Chen, T.; Quan, G.; Ippolito, J.A.; Liu, B.; Yan, J.; Ding, C.; Hussain, Q.; Umer, M. Remediation of organic halogen- contaminated wetland soils using biochar. Sci. Total Environ. 2019, 696, 134087.
- Odedishemi, F.O.; Wang, H.-C.; Guadie, A.; Ajibade, T.F.; Fang, Y.-K.; Sharif, H.M.A.; Liu, W.-Z.; Wang, A.-J. Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Bioresour. Technol. 2021, 322, 124430.
- Deng, C.; Huang, L.; Liang, Y.; Xiang, H.; Jiang, J.; Wang, Q.; Hou, J.; Chen, Y. Response of microbes to biochar strengthen nitrogen removal in subsurface flow constructed wetlands: Microbial community structure and metabolite characteristics. Sci. Total Environ. 2019, 694, 133687.
- Buser, H.-R.; Mueller, M.D. Isomer and Enantioselective Degradation of Hexachlorocyclohexane Isomers in Sewage Sludge under Anaerobic Conditions. Environ. Sci. Technol. 1995, 29, 664–672.
- Wypych, G. Handbook of Solvents; ChemTec Publishing: Scarborough, ON, Canada, 2001.
- Arey, J.S.; Green, W.H.; Gschwend, P.M. The Electrostatic Origin of Abraham’s Solute Polarity Parameter. J. Phys. Chem. B 2005, 109, 7564–7573.
- Lamarche, O.; Platts, J.A.; Hersey, A. Theoretical prediction of the polarity/polarizability parameter π2H. Phys. Chem. Chem. Phys. 2001, 3, 2747–2753.
- Chen, W.; Wei, R.; Ni, J.; Yang, L.; Qian, W.; Yang, Y. Sorption of chlorinated hydrocarbons to biochars in aqueous environment: Effects of the amorphous carbon structure of biochars and the molecular properties of adsorbates. Chemosphere 2018, 210, 753–761.
- Marcus, Y. Linear solvation energy relationships. Correlation and prediction of the distribution of organic solutes between water and immiscible organic solvents. J. Phys. Chem. 1991, 95, 8886–8891.
- Yang, K.; Wu, W.; Jing, Q.; Zhu, L. Aqueous Adsorption of Aniline, Phenol, and their Substitutes by Multi-Walled Carbon Nanotubes. Environ. Sci. Technol. 2008, 42, 7931–7936.
- Murthy, H.M.R.; Manonmani, H.K. Aerobic degradation of technical hexachlorocyclohexane by a defined microbial consortium. J. Hazard. Mater. 2007, 149, 18–25.
- Liu, X.; Peng, P.; Fu, J.; Huang, W. Effects of FeS on the Transformation Kinetics of γ-Hexachlorocyclohexane. Environ. Sci. Technol. 2003, 37, 1822–1828.
- Liu, J.; Wang, Z.; Belchik, S.M.; Edwards, M.J.; Liu, C.; Kennedy, D.W.; Merkley, E.D.; Lipton, M.S.; Butt, J.N.; Richardson, D.J.; et al. Identification and Characterization of MtoA: A Decaheme c-Type Cytochrome of the Neutrophilic Fe(II)-Oxidizing Bacterium Sideroxydans lithotrophicus ES-1. Front. Microbiol. 2012, 3, 37.
- Dadhwal, M.; Singh, A.; Prakash, O.; Gupta, S.; Kumari, K.; Sharma, P.; Jit, S.; Verma, M.; Holliger, C.; Lal, R. Proposal of biostimulation for hexachlorocyclohexane (HCH)-decontamination and characterization of culturable bacterial community from high-dose point HCH-contaminated soils. J. Appl. Microbiol. 2009, 106, 381–392.
- Leoni, C.; Volpicella, M.; Fosso, B.; Manzari, C.; Piancone, E.; Dileo, M.C.G.; Arcadi, E.; Yakimov, M.; Pesole, G.; Ceci, L.R. A Differential Metabarcoding Approach to Describe Taxonomy Profiles of Bacteria and Archaea in the Saltern of Margherita di Savoia (Italy). Microorganisms 2020, 8, 936.
- Miyauchi, K.; Suh, S.-K.; Nagata, Y.; Takagi, M. Cloning and Sequencing of a 2,5-Dichlorohydroquinone Reductive Dehalogenase Gene Whose Product Is Involved in Degradation of γ-Hexachlorocyclohexane by Sphingomonas paucimobilis. J. Bacteriol. 1998, 180, 1354–1359.
- Nagata, Y.; Nariya, T.; Ohtomo, R.; Fukuda, M.; Yano, K.; Takagi, M. Cloning and sequencing of a dehalogenase gene encoding an enzyme with hydrolase activity involved in the degradation of gamma-hexachlorocyclohexane in Pseudomonas paucimobilis. J. Bacteriol. 1993, 175, 6403–6410.
