The hemorrhagic syndrome is one of the most serious complications in patients who have been in contact with the Lonomia caterpillar bristles. Although 26 species of the genus Lonomia (Saturniidae family) are distributed in the American continent, the most studied species are L. obliqua and L. achelous caterpillars; both are capable of inducing hemorrhagic effects in humans. Envenoming by L. obliqua caterpillars was considered a public health problem in southern Brazil. The hemostatic disturbances observed in the envenoming by L. obliqua caterpillars, result in a consumption coagulopathy, resembling a disseminated intravascular coagulation (DIC) and secondary fibrinolysis, which can lead to the hemorrhagic syndrome. The main complication of L. obliqua envenomation is acute renal failure, which can occur in up to 12% of the cases, being frequent in patients over 45 years old and in those with heavy bleeding. Besides that, some deaths related to hemorrhage and renal failure have been reported. However, the early diagnosis and proper treatment with antilonomic serum (ALS), produced by the Butantan Institute (SP/Brazil), within 12 h of contact can prevent severe coagulopathy and hemorrhage events.
. Introduction
Although lepidopteran species are widely distributed around the world, only a few of them cause severe damage to humans or animals that have had contact with adult animal hairs (lepidopterism) or with the bristles of caterpillars (erucism) [
1]. Locally, accidental contact with hair or bristles leads to a skin reaction, and systemic symptoms can be treated using oral antipruritic and antihistamines [
2]. However, some caterpillar species of the
Lonomia genus cause serious injuries, which are sometimes irreversible, leading to death. Patients that develop clinical manifestations of disseminated intravascular coagulation (DIC) and consumptive coagulopathy can progress to hemorrhagic syndrome with serious consequences if the antilonomic serum (ALS) produced by the Butantan Institute (SP/Brazil) is not administered in due time [
3,
4,
5,
6,
7,
8]. Although treatment with ALS is effective for
Lonomia’s envenoming, deaths resulting from contact with caterpillars are still a public health problem in Brazil [
9,
10]. The literature reports that, between 2007 and 2017 a total of 42,264 accidents were caused by caterpillars in Brazil, among them 248 were severe cases and five evolved to deaths. Most accidents occurred in the states of south and southern Brazil between December and April, a period corresponding to an increase in temperature and rainfall [
10].
Over the years, Brazil has gained significant knowledge in the field of toxinology that benefits the politics of public health. One example is the existence of Toxicological Information and Assistance Centers (CIATox), created for the Brazilian Unified Health System (SUS) to provide specific information on poisoning and treatment to health professionals and to the community. Furthermore, the creation of special programs and centers for research in the study of animal toxins contributed to the innovation in the development of new molecules derived from animal toxins or secretions, accelerating the interaction between science and industry. Therefore, this review highlights the current knowledge about Lonomia envenoming, as well as its treatment and already identified bioactive molecules, approaching the future perspectives on innovative research with new derived compounds as potential drugs for the treatment of inflammatory diseases (Figure 1).
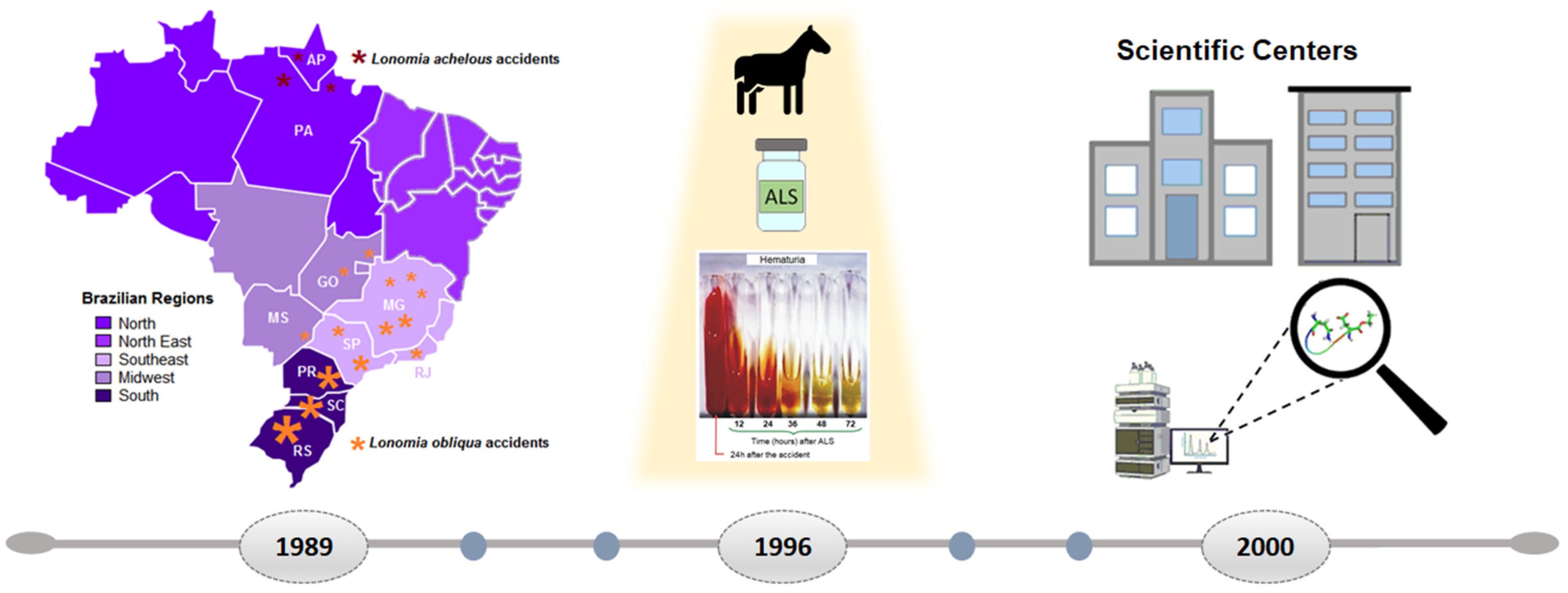
Figure 1. Overview of
Lonomia obliqua epidemiology, treatment, and research over the years. Since 1989, a burst of accidents with hemorrhagic manifestations were reported in Brazil to be caused by
L. obliqua (Walker, 1855) (orange asterisks), mainly in Santa Catarina (SC), Rio Grande do Sul (RS), and Paraná (PR). In Venezuela and northern Brazil [Amapá (AP) and Pará (PA)], caterpillars were identified as
L. achelous (Cramer) (red asterisks). Other cases were registered in the states of Goiás (GO), Minas Gerais (MG), São Paulo (SP), Mato Grosso do Sul (MS), and Rio de Janeiro (RJ) (orange asterisks). In 1996, an antivenom against
L. obliqua toxins was developed [
3]. Today, the treatment of patients is based on the administration of ALS, produced at the Butantan Institute, which has been shown to be effective in reversing hemostatic and hemorrhagic disorders. Photograph (Dr. Marlene Zannin) showing the reduction in hematuria of urine samples of a patient with treatment started with ALS after 24 h of having an accident with
L. obliqua caterpillars. In 2000, the State of São Paulo Research Foundation (FAPESP) started a program to create Research, Innovation, and Dissemination Centers (RIDC) leading to the creation of the “Center for Applied Toxinology (CAT)”, the “Center of Toxins, Immune Response, and Cell Signaling (CETICS)”, and the “Center of Excellence in New Target Discovery (CENTD)”, with the latter aiming at not only the study of toxins from poisons and animal secretions, but also the development of new molecules based on toxins and, in public–private partnerships, their use as tools for studying molecular targets for several diseases.
2. Effect of LOCBE and Toxins in the Inflammatory Response
Dermatitis and skin reactions such as urticaria are well-known signs after accidental contact with the spines and bristles of venomous lepidopteran caterpillars [
1]. Generally, the consequences of these reactions are limited to local inflammation, with no systemic or tissue damage. In the case of the envenomation caused by
L. obliqua, the process is characterized by triggering an intense inflammatory response in victims followed by coagulation, complement, and kallikrein–kinin systems [
36,
48,
51]. In recent years, several studies have been carried out aiming to clarify and describe the role of the venom-induced inflammatory response in the clinical symptoms characteristic of lonomism.
L. obliqua proinflammatory effects are first manifested by pain, burning sensation, edema, and erythema formation [
8,
19,
28]. The first pharmacological studies showed that venom-induced nociception in animal models is largely facilitated by the production of prostaglandins, and later edematogenic symptoms are induced by prostanoids and histamines [
48,
71]. The kallikrein–kinin system is also involved in the edematogenic and hypotensive responses triggered by the venom. Bohrer and collaborators demonstrated that administration, prior to treatment with LOCBE, of plasma kallikrein inhibitor reduces the volume of venom-induced edema in a mouse paw model [
35].
Envenomed patients presented low levels of plasma prekallikrein [
8,
19], indicating that kallikrein was activated and released into the blood circulation. The kallikrein–kinin system is composed of proteolytic enzymes and their substrates, being able to generate potent vasoactive and proinflammatory molecules that are involved in the control of blood pressure, vascular permeability, vascular smooth muscle cell contraction or relaxation, and pain [
86]. One of the common consequences of lonomism is the sudden loss of basic renal functions. Kidneys and urine from envenomed animals were enriched with proteins related to inflammatory stress, tissue damage, oxidative stress, coagulation, complement system activation, and kinin system [
87,
88]. When simultaneously treated with kallikrein inhibitors and antilonomic serum, envenomed rats showed improvements in renal and vascular function, reducing tubular necrosis and renal inflammation [
32]. The mechanism underlying these effects was associated with lowering renal inflammation, with a decrease in proinflammatory cytokines and matrix metalloproteinase expression, reduced tubular degeneration, and protection against oxidative stress.
An increase in the permeability of the endothelium allows greater infiltration of cells of the immune system, effectors, and regulators of acute inflammation into tissues [
89]. Increased vascular tissue permeability is a characteristic event of the inflammatory response and can be induced by several proinflammatory and vasoactive substances such as bradykinin, histamine, thrombin, cytokines, prostaglandins, and free radicals [
74,
90]. Activation of the vascular tissue was observed after a single subcutaneous injection of LOCBE in rats [
91]. Envenomed animals demonstrated neutrophilic leukocytosis in several tissues, where their histological sections provided evidence of inflammatory cell infiltrates in the heart, lungs, and kidneys, characterizing a systemic acute inflammatory response induced by the venom [
92]. Furthermore, an increase was observed in leukocyte rolling and adhesion of these circulating blood cells to the endothelium of hamster cheek pouch tissue that was previously incubated with low doses of LOCBE [
72]. The ability of LOCBE to induce an increase in the permeability of the vasculature and immune cell infiltration may provide a favorable environment for hemorrhages, especially in microvessels in the brain.
Due to the important relationship between inflammation and vasculature, studies were carried out seeking to elucidate the direct effect of LOCBE on vascular tissue. In vitro studies showed that non-hemorrhagic concentrations of LOCBE modify the cytoskeleton dynamics and increase focal adhesion in endothelial cells [
72]. Furthermore, low doses of the LOCBE can induce activation of the nuclear transcription factor κB (NF-κB) pathway in these cells [
73]. The NF-κB pathway is a critical signaling in several events associated with triggering acute inflammation and immune system cell recruitment [
93]. Consequently, LOCBE also induced significant increases in the expression of COX-2, NOS-2, HO-1, MMP-2, and MMP-9, enzymes related to prostaglandin production, oxidative stress, and extracellular matrix degradation [
72,
74].
Additionally, the LOCBE was also shown to be a potent activator of vascular smooth muscle cells, being able to induce cell chemotaxis, exacerbated proliferation, and production of reactive oxygen species. Smooth muscle cell dysfunction is characterized by increased cell migration and proliferation, events that are amplified by the release of inflammatory mediators [
91]. Furthermore, researchers also carried out a broad analysis of the gene expression profile of fibroblasts treated with LOCBE. The results show an upregulation of several proinflammatory mediator genes, such as IL-8, IL-6, and CCL2, as well as the adhesion molecule ICAM-3 and COX-2 [
74]. Recently, our group showed a direct effect of LOCBE upon macrophage activation. The LOCBE directly induces THP-1 macrophages to a proinflammatory phenotype by activating NF-κB pathway, leading the cells to release proinflammatory cytokines and chemokines such TNFα, IL-1β, IL-6, IL-8, and CXCL10 [
73].
The role of isolated toxins present in the venom triggering inflammatory responses is still to be investigated. In addition to its procoagulant activity, in vivo studies show that injection of a high concentration of recombinant
L. obliqua prothrombin activator protease (rLopap) in rats promotes neutrophil and monocyte infiltration in pulmonary microcirculation vessels. In HUVECs, rLopap stimulates the increase of IL-8, ICAM-1, and E-selectin, proteins involved in the recruitment of immune cells to the tissue [
56,
75]. In contrast, there is no evidence that Losac presents proinflammatory activity beyond its cytoprotective and proliferative effects.
Taken together, the evidence indicates that LOCBE can induce a local acute inflammatory response that can evolve into a systemic response. Many studies have characterized the role of the kinin–kallikrein system and the liberation of other proinflammatory mediators by the affected tissues, related to several clinical stomps. The isolated effect of the toxins present in the LOCBE, such as Lopap, and their roles in the activation of prekallikrein, the ability to directly induce cell responses, and the molecular mechanism underlying these effects still need to be clarified.
3. Concluding Remarks
After the burst of accidents with hemorrhagic manifestations in 1989 in the states of south and southeast Brazil, many efforts were put into finding a solution to the public health problem that reached alarming proportions. Fortunately, the treatment with ALS produced at the Butantan Institute drastically reduced the consequences of the envenoming. All these efforts have resulted in substantial knowledge about the pathophysiology of the envenoming and the toxins contained in the venom.
Over the years, research has diversified, addressing not only the hematological alterations of the venom but also the role of toxins in each observed manifestation (Table 2). New molecules identified demonstrate peculiarities, such as hemolin and lipocalin structures with enzymatic or cellular activities that have never previously been reported for these types of proteins. This opened new perspectives for the use of those molecules in several applications, as diagnostic agents, for example, to detect dysprothrombinemias, as in the case of Lopap, which resulted in several patents granted, or as therapeutic agents for use in defibrinogenating and antithrombotic therapy (Lopap) or for promoting wound healing (Lopap- and Losac-derived peptides) (Table 3).
The creation of multidisciplinary research centers specialized in the study of animal envenomation and animal toxins promoted the interaction of partnerships with the pharmaceutical industry, e.g., CENTD, taking the study of molecules derived from venoms and toxins to another level, favoring the development and innovation of new molecules. High-throughput screening technologies for new drugs or target discovery are extensively used at CENTD using toxin-derived peptides as tools for identifying new targets for inflammatory diseases.
Therefore, it is clear that the efforts of distinct groups at Butantan Institute have not only contributed to accumulated knowledge about the toxinology of Lonomia, but also created the basis for the development of ALS, essential in reducing the deaths due to lononism, and prompted the institute to develop innovative initiatives with toxin-derived peptides.
This entry is adapted from the peer-reviewed paper 10.3390/toxins13120832

