Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Obstetrics & Gynaecology
|
Oncology
Female oncofertility is recently implemented conception in the female fertility treatment. It includes the therapeutic approaches to preserve the fertility competence in cancer patients.
- chemotherapy
- fertility preservation
- gonadotoxic
- oncofertility
- oocyte quality
1. Introduction
In the past two decades, substantial advances in early diagnosis and cancer treatment have resulted in an approximately 80% 5-year survival rate in young oncological patients [1][2], leading to a rise in number of female childhood cancer survivors (CCS) [3]. However, oncologic treatment usually requires extensive chemotherapy and/or radiotherapy, which are indicated to be distinctively ovotoxic, resulting in premature ovarian insufficiency (POI) and consequent infertility [4][5][6][7]. Approximately, 30% of children who were exposed to chemo- and/or radio-therapy develop gonadal dysfunction [8]. The incidence of POI in CCS is estimated as high as 8–10% [4].
Although the mechanism is not fully elucidated yet, current data demonstrate that chemotherapeutic agents, especially alkylating ones, interfere with DNA replication and cell division [9], massively activate the primordial follicles (PFs) [10][11], cause stroma atresia [12], and damage the vascularity in ovaries [13]. The radiation is also harmful to oocytes as its low dose of less than 2 Gy can destroy 50% of primordial follicles [7][14].
This fertility-compromised status has been well-documented to cause emotional distress and poor quality of life [15][16][17][18]. It was reported that there will be approximately 100 million women at risk of chemotherapy-induced ovarian impairment in 2025 [19]. In this context, preserving the fertility and quality of life of CCS has received considerable concerns. During the last two decades, FP with several effective approaches has been significantly developed, and represents a beneficial option to help hundreds of oncological women have genetic offspring. Furthermore, increasing studies are attempting to clarify the mechanisms and outcomes of chemo- and radio-therapy impacts on the ovarian reserve and oocyte quality, to develop protective methods as well as to improve therapeutic approaches in FP.
2. Impact of Chemo- and Radio-Therapy on Follicle Quantity
2.1. Clinical Data Describing the Impact of Chemo- and Radio-Therapy on Ovarian Function
A growing number of studies have demonstrated that the pregnancy rate and live birth rate in female CCS are lower compared to those of their siblings and general population controls. The results of these studies are summarized in Table 1.
Table 1. Summary of published clinical studies describing chemo- and radio-therapy on ovarian function.
| Authors | Number of CCS | Age | Exposure Agent a | Radiation | Effects | |
|---|---|---|---|---|---|---|
| Clinical | Laboratory Test | |||||
| Berjeb et al. (2021) [20] | 66 | 15–40 (26.7 ± 6.8) |
Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, doxorubicin, vinblastine, dacarbazine | No | N/A | ↓ AMH |
| Filippi et al. (2021) [21] | 90 | 21.3 ± 5.4 | Bleomycin, cisplatin, bleomycin, dacarbazine-vinblastine | Yes/No b | ↑ POI rate (21% of treated women) | |
| Gini et al. (2019) [22] | 97 | 16–50 (median: 28) |
Doxorubicin, cyclophosphamide, vincristine, bleomycin | Yes | ↑ Amenorrhea | N/A |
| Lehmann et al. (2019) [23] | 444 | ≤40 | N/A | Yes/No | N/A | ↑ LH ↑ FSH ↓ E2 |
| Anderson et al. (2018) [4] | 23,201 | ≤39 | N/A | N/A | ↓ Pregnancy rate (↓ 38%) | |
| Shandley et al. (2018) [24] | 1090 | 20–35 (median: 26) |
N/A | No | N/A | ↓ AFC↓ AMH |
| Sinha et al. (2018) [25] | 88 | 24–43 (median: 35) |
Taxotere, cyclophosphamide, carboplatin, fluorouracil, epirubicin | No | N/A | ↓ AFC |
| Al-Rawi et al. (2018) [26] | 58 | 25–45 (38.83 ± 4.74) |
Anthracycline, cyclophosphamide | No | N/A | ↓ AFC↓ E2 ↑ LH |
| Aderson et al. (2018) [27] | 67 | 18–45 | Doxorubicin, bleomycin, vinblastine, and dacarbazine | No | N/A | ↓ AMH ↑ FSH ↓ E2 |
| Levine et al. (2018) [28] | 2930 | 18–58 (median: 32) |
Alkylating agent, procarbazine | Yes/No | ↑ POI rate (9.1% of treated women) | N/A |
| Armuand et al. (2017) [29] | 552 | ≥13 | N/A | N/A | ↓ The probability of having a first live birth | N/A |
| Chemaitilly et al. (2017) [30] | 988 | 18–45 (median: 31.7) |
Alkylating agents | Yes | ↑ POI rate (10.9% of treated women) | N/A |
| D’Avila et al. (2017) [31] | 52 | 27–40 (35.3 ± 3.8) |
Cyclophosphamide | No | ↑ Amenorrhea | ↓ AFC↓ AMH ↑ FSH |
| Abir et al. (2016) [32] | 20 | 5–18 | Alkylating agents, bleomycin, cisplatin, vincristine, etoposide, carboplatin, doxorubicin, etopside, doxorubicin, bleomycin, vinblastine, dacarbazine. | No | ↑ Atretic follicles↓ Oocyte maturation | N/A |
| Hamy et al. (2016) [33] | 134 | 26–43 (median: 36) |
Anthracyclines, taxane | No | N/A | ↓ AMH |
| Even-Or et al. (2016) [34] | 35 | 13–36 (median: 25.5) |
Melphalan | No | N/A | ↓ AMH ↑ FSH ↓ LH |
| Gupta et al. (2016) [35] | 16 | 11–18 (median: 14.3) |
Doxorubicin, cyclophosphamide, cisplatin | No | ↑ Amenorrhea | ↓ AMH |
| Chow et al. (2016) [5] | 5298 | 15–44 | Busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide | Yes/No | ↓ Pregnancy rate | N/A |
| Thomas-Teinturier et al. (2015) [36] | 105 | 18–39 (median: 21.5) |
Cyclophosphamide, ifosfamide | Yes | N/A | ↓AMH ↑ FSH |
| Behringer et al. (2012) [37] | 106 | 18–39 (28 ± 7) |
Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, doxorubicin, bleomycin, vinblastine, dacarbazine | N/A | N/A | ↓ AMH ↑ FSH |
| Green et al. (2009) [38] | 5149 | 15–44 | Alkylating agents | Yes/No | ↓ Pregnancy rate | N/A |
a: All chemotherapeutic agents exposed that all included patients were exposed to are listed in each study. b: Some patients treated by both radiation and chemotherapy, but some patients were treated only with chemotherapy. ↓: Decreased. ↑: Increased. AFC: antral follicle count, AMH: anti-Müllerian hormone, E2: estradiol, FSH: follicle-stimulating hormone, LH: luteinizing hormone, N/A: not available or not applicable, POI: premature ovarian insufficiency.
In a longitudinal study including 66 patients undergoing chemotherapy, the AMH levels were decreased significantly (0.90 ± 1.55 compared to 2.61 ± 2.20 ng/mL before treatment) after chemotherapy using BEACOPP protocol (Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine, and Prednisolone) during a following period of 16.8 ± 9.3 months. In the ABVD protocol (Doxorubicin, Bleomycin, Vinblastine, and Dacarbazine), the AMH levels prior and after treatment were not statistically different (4.38 ± 3.39 vs. 4.27 ± 3.09 ng/mL, p = 0.753) [20]. Another study recorded the rates of diminished ovarian reserve and POI after chemotherapy as 39% (35/90) and 21% (19/90), respectively [21].
According to a population-based analysis, the overall likelihood of pregnancy in female CCSs aged under 40 is a 38% lower than that in the general population of women [4]. In another study, laboratory results show impairment in the concentration of female gonadal-related hormones (LH, FSH, and estradiol) in 24.3% (97/444) of female CCSs who were younger than 40 years of age [23]. According to a cohort study of 552 female CCSs in Sweden, the hazard ratio (HR) for having a first live birth in CCSs with malignancy of the eye, central nervous system tumors, and leukemia, is statistically lower than in healthy controls [29]. In 2930 CCSs, 110 survivors encountered POI with the value of 10.3 as an odds ratio compared to their healthy siblings, resulting in lower birth rates in their thirties [28]. In another report, the relative likelihood of 5149 CCSs achieving pregnancy is 0.81 (95% CI, 0.73 to 0.90; p < 0.001) compared with that of female siblings [38]. In a large sample cohort study including 5298 female five-year cancer survivors, their likelihood of having a pregnancy is significantly lower than their siblings (HR 0.85, 95%: 0.74–0.98; p = 0.023) [5]. The effects of the alkylating drugs and cisplatin on ovarian functions show a dose-dependent manner [5]. In a systematic review including 5607 female CCSs, the prevalence of amenorrhea ranges from 0% to 83% [6]. Exposure to alkylating agents and older age at treatment are detected as the decisive factors contributing to ovarian dysfunction [6].
2.2. Mechanism of Chemo- and Radio-Therapy Induction of Follicular Loss
To develop new therapies of FP and fertoprotective agents, numerous studies have described the possible mechanisms in which chemo- and radio-therapy induce ovarian damage. As typical chemotherapy protocols often consist of several agents, determining the ovarian impairment caused by each type of antitumor drug in clinical studies is challenging. Consequently, the conceptual effect of a single drug on the ovary is usually clarified by in vitro cell culture, ovarian tissue culture, or in vivo animal models, and human ovarian tissue culture followed by xenotransplantation [39][40].
Chemotherapeutic agents are generally divided into five categories: alkylating agents (cyclophosphamide, procarbazine, and busulfan), platinum-based compounds (cisplatin and carboplatin), anthracycline antibiotics (doxorubicin and bleomycin), antimetabolites (methotrexate and 5-fluorouracil), and vinca alkaloids (vincristine and vinblastine). The first three groups are demonstrated to damage ovaries by inducing DNA alterations, leading to follicular apoptosis [41][42]. Among these, alkylating agents are supposed to be most ovotoxic, causing significant follicular loss [6][43]. The last two groups are indicated to have a low risk to ovarian function [42]. However, some data show that vinca alkaloids, due to their suppression of microtubule dynamics, induce a vascular impairment, leading to ovarian dysfunction [44][45]. Three major mechanisms were proposed by several scientific groups (Figure 1).
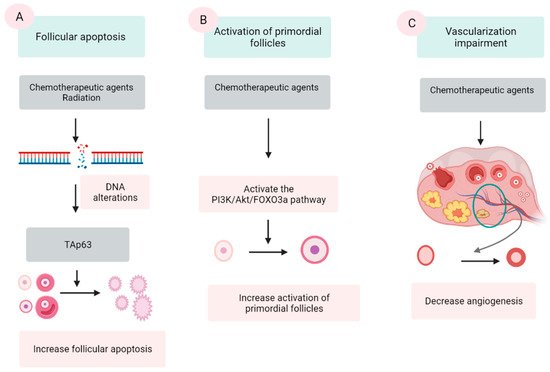
Figure 1. Three mechanisms of chemo- and radio-therapy-induced follicular quantity depletion: enhancement of apoptosis, accelerated activation of PFs. (A) DNA alterations induced by chemotherapeutic agents and radiation activates TAp53 protein, leading to the apoptosis. (B) Chemotherapeutic agents activate the phosphoinositide 3-kinase (PI3K)/Akt/forkhead box protein O3a (FOXO3a), which in turn induce the activation of PFs, resulting in the extensive loss of PFs. (C) Chemotherapeutic agents impair the epithelial tissue of vessels in the ovary, resulting in a reduction in the vascularization.
2.2.1. Follicular Apoptosis after Chemotherapy
The extensive apoptosis of ovarian follicles, especially PFs, after DNA alterations and/or oxidative stress is the most commonly described mechanism in chemotherapy-induced ovarian failure [41][46][47]. Several agents in the antitumor protocols, especially alkylating agents, are demonstrated to cause DNA lesions in both oocyte and granulosa cells (GCs). Among these lesions, double-stranded DNA breaks are among the most severe [39][42]. The accumulation of DNA strand breaks that could not be repaired by the DNA repairing system induces the apoptotic intracellular pathways, resulting in cellular apoptosis [42]. p63 protein (TAp63 isoform), Bcl2-associated X (BAX) protein and the BCL-2 antagonist killer (BAK) protein activator, is the major protein that mediate this mechanism [48][49].
Culture with cyclophosphamide [46] as well as in vivo cyclophosphamide injection [50] of mice’s ovaries induces DNA damage and subsequent follicle apoptosis. Cisplatin also causes DNA impairment and PFs’ apoptosis in both newborn and adult mouse ovaries [41]. Cyclophosphamide treatment substantially decreases the number of PFs, primary follicles, and secondary follicles with an elevated number of atretic follicles compared with control animals [13]. In another experiment, intraperitoneal injection of cyclophosphamide and cisplatin caused a significantly destructive effect on the PFs pool [51]. However, in mice with gene deletion of PUMA, a member of BCL-2 protein family, the PFs are retained after the treatment by both cyclophosphamide and cisplatin [51]. In a human ovarian xenograft model, cyclophosphamide [52], cisplatin [53], and doxorubicin [39] elevated DNA double-stranded breaks and resulted in a significant follicle loss.
The effect of antitumoral drugs on ovarian function is the follicle-specific magnitude and is associated with the category of the drugs [12]. Some studies have declared that apoptosis occurred only in GCs of growing follicles, but not in PFs by TUNEL staining after treatment of cyclophosphamide or cisplatin [54][55]. Other results insist that TUNEL and/or γH2AX staining are positive in oocytes but not in the GCs of PFs [46][50][56]. In another experiment, culturing ovaries with cisplatin or carboplatin decreases the number of follicles of all stages, but the most obvious reduction is observed in PFs [57]. One study reported that culturing of neonatal mice ovaries in cisplatin or doxorubicin significantly decreased the number of follicles at all stages [58]. However, the apoptosis evidence in the TUNEL analysis is not positive in the PFs, only in the growing follicles [58].
2.2.2. Activation of PFs Induced by Chemotherapy
An additional suggested mechanism for ovarian impairment after oncological treatment is the accelerated activation of PFs. Several scientific groups have confirmed that chemotherapy causes massive activation of PFs in affected ovaries via a phosphoinositide 3-kinase/protein kinase B/forkhead box protein O3a (PI3K/AKT/FOXO3a) pathway [54][59][60][61].
Mice administered intraperitoneally with cisplatin show a substantially decreased number of PFs along with higher numbers of early growing follicles and the signal of the key proteins in the PTEN/Akt/FOXO3a [59]. Other studies also revealed increased phosphorylation of Akt, mTORC, and downstream proteins followed by PF reduction in cyclophosphamide-treated mice [54][62]. In mice, doxorubicin causes detrimental effects on ovaries through both atresia and overactivation in PFs [63]. The same effects are found in mice treated with cisplatin [59]. In another experiment using neonatal mouse ovaries cultured with cisplatin or doxorubicin, PFs decrease without the evidence of apoptosis in TUNEL analysis, suggesting the etiology of PF reduction by overactivation [58]. In terms of human ovarian follicles, exposure to cyclophosphamide metabolites in vitro also induces PFs’ activation [61]. Furthermore, a cohort study of 96 female CCSs who were treated with alkylating agent revealed PFs activation in vivo and a remarkably suppressed nuclear expression of FOXO3a occurring in ovaries of these patients [64].
In consistence with this hypothesis, many experiments have indicated that inhibiting the PI3K pathway by several agents including rapamycin, ammonium trichloro (dioxoethylene-o,o′) tellurate (AS101), anti-Müllerian hormone (AMH), and melatonin, could prevent PF’s activation after chemotherapy [54][55][60][65][66][67][68].
Although this mechanism has been widely accepted, recent literature has raised the argument that activating PFs might not be the major or a specific cause of chemotherapy-induced PF loss [11]. Accordingly, the authors doubt that a growing follicles to PFs ratio calculation were not the correct parameter for a sign of PFs’ activation, because elimination of PFs could occur due to a deleterious effect. In an experiment, after culture of intact mouse ovaries with the metabolite agent cyclophosphamide, the number of PFs decreased along with increased levels of apoptotic markers BAX and cPARP. Meanwhile, there was no significant change in the number of primary follicles. In combination with the TUNEL staining’s results, this study indicated that the decrease in PFs was not due to their activation but the apoptosis in PFs [46]. A recent study demonstrated the depletion of PFs after cyclophosphamide exposure in a human ovarian xenograft model, utilizing triggering of proapoptotic pathways without evidence of PFs activation, and indicated that apoptosis was the main mechanism of PFs’ depletion [69].
2.2.3. Vascularization Impairment
Another proposed mechanism is the alteration in angiogenesis and stroma supporting the gonadal cells after exposure to chemotherapeutic agents [70][71]. Cyclophosphamide treatment shows induction of inflammation and enhanced expression of stromal cell-derived factor 1 (SDF-1), a factor related to follicular atresia, which presents in the granulosa, theca cells, and luteinized cells [72]. In human ovaries, histological analyses of ovaries from cancer survivors show the presence of damaged cortical blood vessels and proliferation of small vessels (neovascularization). Furthermore, the muscular layer in blood vessels becomes thicker, leading to limited blood circulation. The cortex presents fibrotic focal areas along with disappearance of follicles [73]. During in vivo monitoring, an evident reduction in ovarian circulation and spasm of small vessels are noted after the administration of doxorubicin [74]. In vitro human ovarian tissue culture with doxorubicin followed by xenograft to immunodeficient mice has a lower vascular density and higher microvascular compromise compared with controls [39]. In addition, one study assessing human ovarian tissue shows that both alkylating and nonalkylating drugs affect ovarian stromal function, leading to a substantial decrease in estradiol production [75].
2.2.4. Radiation
Regarding radiotherapy, the human oocyte is very sensitive to radiation, and a dose as low and less than 2 Gy for pelvic radiation can destroy 50% of PFs [76]. The position of radiation is one determinant factor of the degree of ovarian damage. The rate of POI in patients who experienced total body radiation and pelvic irradiation are 90% and 97%, respectively [77]. In addition, factors such as patient age and radiation dose are also important contributors [73][77]. Aging patients are more vulnerable to radiation compared with younger girls, due to the age-related decline in the follicle population [78]. The dose causing ovarian dysfunction in children is 1–2 Gy, whereas in adults it is as low as 0.4–0.6 Gy [79]. Based on an analysis from five centers conducting ovarian tissue cryopreservation (OTC), the live birth rates after OTC in patients undergoing pelvic irradiation reduced significantly in a dose-dependent manner [80].
The proposed mechanism of follicle depletion is the radiation-provoked ionizing damage of DNA [81]. This alteration also activates TAp63 protein, leading to destruction of PFs [49]. In terms of late effects, vasculature damage and stromal fibrosis following tissue hypoxia could be another mechanism [77]. This can result in ovarian atrophy and subsequent tissue dysfunction [73].
This entry is adapted from the peer-reviewed paper 10.3390/jcm10235690
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34.
- Trama, A.; Bernasconi, A.; McCabe, M.G.; Guevara, M.; Gatta, G.; Botta, L.; Ries, L.; Bleyer, A. Is the cancer survival improvement in European and American adolescent and young adults still lagging behind that in children? Pediatr. Blood Cancer 2019, 66, e27407.
- Trama, A.; Botta, L.; Foschi, R.; Ferrari, A.; Stiller, C.; Desandes, E.; Maule, M.M.; Merletti, F.; Gatta, G. Survival of European adolescents and young adults diagnosed with cancer in 2000–07: Population-based data from EUROCARE-5. Lancet Oncol. 2016, 17, 896–906.
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290.
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016, 17, 567–576.
- Overbeek, A.; van den Berg, M.H.; van Leeuwen, F.E.; Kaspers, G.J.; Lambalk, C.B.; van Dulmen-den Broeder, E. Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat. Rev. 2017, 53, 10–24.
- Anderson, R.A.; Mitchell, R.T.; Kelsey, T.W.; Spears, N.; Telfer, E.E.; Wallace, W.H. Cancer treatment and gonadal function: Experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015, 3, 556–567.
- Pampanini, V.; Hassan, J.; Oliver, E.; Stukenborg, J.B.; Damdimopoulou, P.; Jahnukainen, K. Fertility Preservation for Prepubertal Patients at Risk of Infertility: Present Status and Future Perspectives. Horm. Res. Paediatr. 2020, 93, 599–608.
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535.
- Sonigo, C.; Beau, I.; Binart, N.; Grynberg, M. The Impact of Chemotherapy on the Ovaries: Molecular Aspects and the Prevention of Ovarian Damage. Int. J. Mol. Sci. 2019, 20, 5342.
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566.
- Yuksel, A.; Bildik, G.; Senbabaoglu, F.; Akin, N.; Arvas, M.; Unal, F.; Kilic, Y.; Karanfil, I.; Eryılmaz, B.; Yilmaz, P.; et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum. Reprod. 2015, 30, 2926–2935.
- Pascuali, N.; Scotti, L.; Di Pietro, M.; Oubiña, G.; Bas, D.; May, M.; Muñoz, A.G.; Cuasnicú, P.S.; Cohen, D.J.; Tesone, M.; et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 2018, 33, 844–859.
- Wallace, W.H.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat Oncol. Biol. Phys. 2005, 62, 738–744.
- Duffy, C.; Allen, S. Medical and psychosocial aspects of fertility after cancer. Cancer J. 2009, 15, 27–33.
- Niedzwiedz, C.L.; Knifton, L.; Robb, K.A.; Katikireddi, S.V.; Smith, D.J. Depression and anxiety among people living with and beyond cancer: A growing clinical and research priority. BMC Cancer 2019, 19, 943.
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405.
- Logan, S.; Perz, J.; Ussher, J.M.; Peate, M.; Anazodo, A. Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology 2019, 28, 22–30.
- Sun, B.; Yeh, J. Onco-fertility and personalized testing for potential for loss of ovarian reserve in patients undergoing chemotherapy: Proposed next steps for development of genetic testing to predict changes in ovarian reserve. Fertil. Res. Pract. 2021, 7, 13.
- Berjeb, K.K.; Debbabi, L.; Braham, M.; Zemni, Z.; Chtourou, S.; Hannachi, H.; Hamdoun, M.; Ayadi, M.; Kacem, K.; Zhioua, F.; et al. Evaluation of ovarian reserve before and after chemotherapy. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102035.
- Filippi, F.; Meazza, C.; Somigliana, E.; Podda, M.; Dallagiovanna, C.; Massimino, M.; Raspagliesi, F.; Terenziani, M. Fertility preservation in childhood and adolescent female tumor survivors. Fertil. Steril. 2021, 116, 1087–1095.
- Gini, G.; Annibali, O.; Lupasco, D.; Bocci, C.; Tomarchio, V.; Sampaolo, M.; Trappolini, S.; Tafuri, M.A.; Cacciagiù, S.; Ciccarone, M.; et al. Gonadal Function Recovery and Fertility in Women Treated with Chemo- and/or Radiotherapy for Hodgkin’s and Non-Hodgkin Lymphoma. Chemotherapy 2019, 64, 36–41.
- Lehmann, V.; Chemaitilly, W.; Lu, L.; Green, D.M.; Kutteh, W.H.; Brinkman, T.M.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Klosky, J.L. Gonadal Functioning and Perceptions of Infertility Risk Among Adult Survivors of Childhood Cancer: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2019, 37, 893–902.
- Shandley, L.M.; Fothergill, A.; Spencer, J.B.; Mertens, A.C.; Cottrell, H.N.; Howards, P.P. Impact of cancer treatment on risk of infertility and diminished ovarian reserve in women with polycystic ovary syndrome. Fertil. Steril. 2018, 109, 516–525.e511.
- Sinha, N.; Letourneau, J.M.; Wald, K.; Xiong, P.; Imbar, T.; Li, B.; Harris, E.; Mok-Lin, E.; Cedars, M.I.; Rosen, M.P. Antral follicle count recovery in women with menses after treatment with and without gonadotropin-releasing hormone agonist use during chemotherapy for breast cancer. J. Assist. Reprod. Genet. 2018, 35, 1861–1868.
- Al-Rawi, S.A.; Saleh, B.O.; Al-Naqqash, M.A. Serum anti-müllerian hormone levels in evaluation of chemotherapy effect on ovarian reserve in women with breast cancer. A follow-up study. Saudi Med. J. 2018, 39, 733–735.
- Anderson, R.A.; Remedios, R.; Kirkwood, A.A.; Patrick, P.; Stevens, L.; Clifton-Hadley, L.; Roberts, T.; Hatton, C.; Kalakonda, N.; Milligan, D.W.; et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’s lymphoma (RATHL): A secondary analysis of a randomised phase 3 trial. Lancet Oncol. 2018, 19, 1328–1337.
- Levine, J.M.; Whitton, J.A.; Ginsberg, J.P.; Green, D.M.; Leisenring, W.M.; Stovall, M.; Robison, L.L.; Armstrong, G.T.; Sklar, C.A. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2018, 124, 1044–1052.
- Armuand, G.; Skoog-Svanberg, A.; Bladh, M.; Sydsjö, G. Reproductive Patterns Among Childhood and Adolescent Cancer Survivors in Sweden: A Population-Based Matched-Cohort Study. J. Clin. Oncol. 2017, 35, 1577–1583.
- Chemaitilly, W.; Li, Z.; Krasin, M.J.; Brooke, R.J.; Wilson, C.L.; Green, D.M.; Klosky, J.L.; Barnes, N.; Clark, K.L.; Farr, J.B.; et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors: A Report From the St. Jude Lifetime Cohort. J. Clin. Endocrinol. Metab. 2017, 102, 2242–2250.
- D’Avila, Â.M.; Capp, E.; Corleta, H.V.E. Antral Follicles Count and Anti-Müllerian Hormone Levels after Gonadotoxic Chemotherapy in Patients with Breast Cancer: Cohort Study. Rev. Bras. Ginecol. Obstet. 2017, 39, 162–168.
- Abir, R.; Ben-Aharon, I.; Garor, R.; Yaniv, I.; Ash, S.; Stemmer, S.M.; Ben-Haroush, A.; Freud, E.; Kravarusic, D.; Sapir, O.; et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016, 31, 750–762.
- Hamy, A.S.; Porcher, R.; Eskenazi, S.; Cuvier, C.; Giacchetti, S.; Coussy, F.; Hocini, H.; Tournant, B.; Perret, F.; Bonfils, S.; et al. Anti-Müllerian hormone in breast cancer patients treated with chemotherapy: A retrospective evaluation of subsequent pregnancies. Reprod. Biomed. Online 2016, 32, 299–307.
- Even-Or, E.; Ben-Haroush, A.; Yahel, A.; Yaniv, I.; Stein, J. Fertility After Treatment With High Dose Melphalan in Women With Acute Myelogenous Leukemia. Pediatr. Blood Cancer 2016, 63, 334–336.
- Gupta, A.A.; Chong, A.L.; Deveault, C.; Traubici, J.; Maloney, A.M.; Knight, S.; Lorenzo, A.; Allen, L. Anti-Müllerian Hormone in Female Adolescent Cancer Patients Before, During, and After Completion of Therapy: A Pilot Feasibility Study. J. Pediatr. Adolesc. Gynecol. 2016, 29, 599–603.
- Thomas-Teinturier, C.; Allodji, R.S.; Svetlova, E.; Frey, M.A.; Oberlin, O.; Millischer, A.E.; Epelboin, S.; Decanter, C.; Pacquement, H.; Tabone, M.D.; et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum. Reprod. 2015, 30, 1437–1446.
- Behringer, K.; Thielen, I.; Mueller, H.; Goergen, H.; Eibl, A.D.; Rosenbrock, J.; Halbsguth, T.; Eichenauer, D.A.; Fuchs, M.; Reiners, K.S.; et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann. Oncol. 2012, 23, 1818–1825.
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J. Clin. Oncol. 2009, 27, 2677–2685.
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793.
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; De Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113.
- Gonfloni, S.; Di Tella, L.; Caldarola, S.; Cannata, S.M.; Klinger, F.G.; Di Bartolomeo, C.; Mattei, M.; Candi, E.; De Felici, M.; Melino, G.; et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat. Med. 2009, 15, 1179–1185.
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344.
- Donnez, J.; Dolmans, M.M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665.
- Lambertini, M.; Olympios, N.; Lequesne, J.; Calbrix, C.; Fontanilles, M.; Loeb, A.; Leheurteur, M.; Demeestere, I.; Di Fiore, F.; Perdrix, A.; et al. Impact of Taxanes, Endocrine Therapy, and Deleterious Germline BRCA Mutations on Anti-müllerian Hormone Levels in Early Breast Cancer Patients Treated With Anthracycline- and Cyclophosphamide-Based Chemotherapy. Front. Oncol. 2019, 9, 575.
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803.
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256.
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase II mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18.
- Kerr, J.B.; Hutt, K.J.; Michalak, E.M.; Cook, M.; Vandenberg, C.J.; Liew, S.H.; Bouillet, P.; Mills, A.; Scott, C.L.; Findlay, J.K.; et al. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol. Cell 2012, 48, 343–352.
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134.
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Cisplatin- and cyclophosphamide-induced primordial follicle depletion is caused by direct damage to oocytes. Mol. Hum. Reprod. 2019, 25, 433–444.
- Nguyen, Q.N.; Zerafa, N.; Liew, S.H.; Morgan, F.H.; Strasser, A.; Scott, C.L.; Findlay, J.K.; Hickey, M.; Hutt, K.J. Loss of PUMA protects the ovarian reserve during DNA-damaging chemotherapy and preserves fertility. Cell Death Dis. 2018, 9, 618.
- Oktem, O.; Oktay, K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007, 67, 10159–10162.
- Bildik, G.; Acılan, C.; Sahin, G.N.; Karahuseyinoglu, S.; Oktem, O. C-Abl is not actıvated in DNA damage-induced and Tap63-mediated oocyte apoptosıs in human ovary. Cell Death Dis. 2018, 9, 943.
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra162.
- Xie, Y.; Li, S.; Zhou, L.; Lin, H.; Jiao, X.; Qiu, Q.; Liang, Y.; Zhang, Q. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol. Reprod. Dev. 2020, 87, 442–453.
- Rossi, V.; Lispi, M.; Longobardi, S.; Mattei, M.; Di Rella, F.; Salustri, A.; De Felici, M.; Klinger, F.G. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ. 2017, 24, 72–82.
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. Comparative gonadotoxicity of the chemotherapy drugs cisplatin and carboplatin on prepubertal mouse gonads. Mol. Hum. Reprod. 2020, 26, 129–140.
- Morgan, S.; Lopes, F.; Gourley, C.; Anderson, R.A.; Spears, N. Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PLoS ONE 2013, 8, e70117.
- Chang, E.M.; Lim, E.; Yoon, S.; Jeong, K.; Bae, S.; Lee, D.R.; Yoon, T.K.; Choi, Y.; Lee, W.S. Cisplatin Induces Overactivation of the Dormant Primordial Follicle through PTEN/AKT/FOXO3a Pathway which Leads to Loss of Ovarian Reserve in Mice. PLoS ONE 2015, 10, e0144245.
- Goldman, K.N.; Chenette, D.; Arju, R.; Duncan, F.E.; Keefe, D.L.; Grifo, J.A.; Schneider, R.J. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 3186–3191.
- Lande, Y.; Fisch, B.; Tsur, A.; Farhi, J.; Prag-Rosenberg, R.; Ben-Haroush, A.; Kessler-Icekson, G.; Zahalka, M.A.; Ludeman, S.M.; Abir, R. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod. Biomed. Online 2017, 34, 104–114.
- Chen, X.Y.; Xia, H.X.; Guan, H.Y.; Li, B.; Zhang, W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016, 17, 836.
- Wang, Y.; Liu, M.; Johnson, S.B.; Yuan, G.; Arriba, A.K.; Zubizarreta, M.E.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.; Xiao, S. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol. Appl. Pharmacol. 2019, 381, 114714.
- Shai, D.; Aviel-Ronen, S.; Spector, I.; Raanani, H.; Shapira, M.; Gat, I.; Roness, H.; Meirow, D. Ovaries of patients recently treated with alkylating agent chemotherapy indicate the presence of acute follicle activation, elucidating its role among other proposed mechanisms of follicle loss. Fertil. Steril. 2021, 115, 1239–1249.
- Zhou, L.; Xie, Y.; Li, S.; Liang, Y.; Qiu, Q.; Lin, H.; Zhang, Q. Rapamycin Prevents cyclophosphamide-induced Over-activation of Primordial Follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J. Ovarian Res. 2017, 10, 56.
- Roness, H.; Kashi, O.; Meirow, D. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 2016, 105, 20–29.
- Jang, H.; Hong, K.; Choi, Y. Melatonin and Fertoprotective Adjuvants: Prevention against Premature Ovarian Failure during Chemotherapy. Int. J. Mol. Sci. 2017, 18, 1221.
- Roness, H.; Spector, I.; Leichtmann-Bardoogo, Y.; Savino, A.M.; Dereh-Haim, S.; Meirow, D. Pharmacological administration of recombinant human AMH rescues ovarian reserve and preserves fertility in a mouse model of chemotherapy, without interfering with anti-tumoural effects. J. Assist. Reprod. Genet. 2019, 36, 1793–1803.
- Titus, S.; Szymanska, K.J.; Musul, B.; Turan, V.; Taylan, E.; Garcia-Milian, R.; Mehta, S.; Oktay, K. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci. Rep. 2021, 11, 407.
- Meirow, D.; Epstein, M.; Lewis, H.; Nugent, D.; Gosden, R.G. Administration of cyclophosphamide at different stages of follicular maturation in mice: Effects on reproductive performance and fetal malformations. Hum. Reprod. 2001, 16, 632–637.
- Meirow, D.; Nugent, D. The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 2001, 7, 535–543.
- Luo, Q.; Yin, N.; Zhang, L.; Yuan, W.; Zhao, W.; Luan, X.; Zhang, H. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci. 2017, 179, 103–109.
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633.
- Bar-Joseph, H.; Ben-Aharon, I.; Tzabari, M.; Tsarfaty, G.; Stemmer, S.M.; Shalgi, R. In vivo bioimaging as a novel strategy to detect doxorubicin-induced damage to gonadal blood vessels. PLoS ONE 2011, 6, e23492.
- Oktem, O.; Oktay, K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 2007, 110, 2222–2229.
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121.
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739.
- Wo, J.Y.; Viswanathan, A.N. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1304–1312.
- Ginsberg, J.P. New advances in fertility preservation for pediatric cancer patients. Curr. Opin. Pediatr. 2011, 23, 9–13.
- Dolmans, M.M.; von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115.
- Yasmin, E.; Mitchell, R.; Lane, S. Preservation of fertility in teenagers and young adults treated for haematological malignancies. Lancet Haematol. 2021, 8, e149–e160.
This entry is offline, you can click here to edit this entry!
