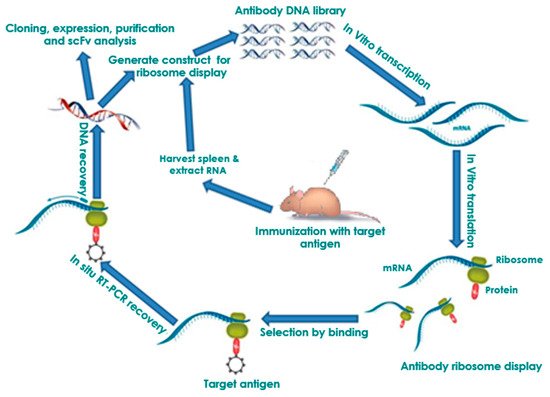
Antibody ribosome display remains one of the most successful in vitro selection technologies for antibodies fifteen years after it was developed. The unique possibility of direct generation of whole proteins, particularly single-chain antibody fragments (scFvs), has facilitated the establishment of this technology as one of the foremost antibody production methods. Ribosome display has become a vital tool for efficient and low-cost production of antibodies for diagnostics due to its advantageous ability to screen large libraries and generate binders of high affinity. The remarkable flexibility of this method enables its applicability to various platforms.
- ribosome display
- • combinatorial libraries
- • biopanning
- • selection
- scFv
- diagnostics
- therapeutics
1. Introduction
2. In Vitro Ribosome Display
2.1. Selection of Antibodies by Panning
2.2. Affinity Maturation and Modification of Ribosome Display Antibodies
2.3. Ribosome Display Antibody Gene Libraries
3. Ribosome Display Technology in Disease Diagnostics and Control
3.1. Human Infectious Diseases
Significant spike mutations (D614G, E484K, K417N/T, N501Y, L452R, T478K) are found associated with different clinical consequences throughout the globe [110]. Scientists observed successful therapeutics from the significant clinical trials, including small antiviral molecules such as remdesivir or antibody-based therapeutics against SARS-CoV-2 [111]. Several antibodies have shown significant neutralization activity against the virus. Some antibodies have received EUA (Emergency Use Authorization) for the treatment of this virus. Most of the antibodies are designed against the S-glycoprotein of this virus. Therefore, any S-glycoprotein mutations can trigger the antibody escapes/antibody resistance in SARS-CoV-2 variants and hinder the antibody-based therapeutic strategies against the virus [110]. To avoid escape mutants, ribosome display technology could possibly be applied to target highly conserved epitopes to produce neutralizing scFv antibody cocktails targeting simultaneously non-RBD and RBD epitopes. Neutralizing antibody cocktails against SARS-CoV-2 would help in the development of diagnostics and treatment for COVID-19.
3.2. Cancer
3.3. Acquired Immunodeficiency Syndrome (AIDS)
3.4. Plant Disease: Pierce’s Disease
3.5. Pain
scFv antibodies are opening a new era of therapeutics, pharmacology, and pathophysiology research [153]. These technologies have overcome previous challenges of providing therapeutic applications for G-protein-coupled receptors (GPCRs). More importantly, these small, brain penetrant antibodies are praised as having promising biotherapeutic applications for the nervous and immune systems, now recognized as interactive in chronic pain. scFvs are being investigated as therapeutics for arthritis, Creutzfeldt-Jakob, and Huntington’s disease due to their solubility, small size, and ability to cross the blood-brain barrier compared to mAbs available for migraine (Galcanezumab, Erenumab) [154-156]. Despite the popularity of scFvs generated by ribosome display for chemotherapy, obtaining high-affinity scFvs from ribosome display libraries remains a challenging task [157].
Chronic pain frequently evokes anxiety, depression, disability, and diminishes quality of life. It is known that cholecystokinin (CCK) evokes anxiety/panic attacks in healthy subjects depending on dosage, and it is 103 times more abundant than any other neuropeptide in the nervous system. Selective antagonists of the CCKB receptor (CCKBR) enhance morphine analgesia and prevent tolerance without worsening respiratory depression in non-human primates or side effects other than orthostatic dizziness in placebo-controlled trials. We have generated a scFv biological that targets mouse CCKBR using ribosome display [158]. The small CCKBR scFv is ~1/6 the size of a monoclonal antibody thus can access the CCKBR biodistribution to positively impact pain circuitry neurons. Its high affinity binding permanently reverses chronic pain-, cognitive-, anxiety-, and depression-related behaviors.
A serious consequence of nerve injury pain or “neuropathic pain” is the transition to chronic pain that remains a significant clinical challenge with a treatment response rate of only 11% [159, 160]. While decades of study have been devoted to acute “nociceptive” mechanisms, it is clear that complex, multifactorial mechanisms are responsible for maintaining neuropathic pain long term, referred to as the “chronification” of pain. Current understanding is pain chronification causes physiological, molecular, epigenetic, and brain circuitry changes. While most studies are done in acute pain models, we utilize clinically relevant models of chronic neuropathic pain and find significantly reduced pain related behaviors after a single treatment with our scFv antibody targeting the P2X4 receptor (P2X4R) using ribosome display [161]. P2X4R upregulation occurs in chronic pain, attributed to microglia in males and to T cells in females [162]. These data provide support for pursuit of P2X4R scFvs as translational therapy for pain relief. We hope to develop non-opioid therapies to treat chronic pain using small protein, brain penetrant, single chain Fragment variable (scFv) antibody therapies. These have the potential to reverse chronic neuropathic pain, associated pain-related behaviors and depression.
New references added:
- Chakraborty, C.; Bhattacharya, M.; Sharma A.R. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape and vaccines activity. Rev Med Virol. 2021, e2270.
- Aleem, A.; AB, A.S.; Slenker A.K. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls Publishing ; January 5, 2022.
- Harvey, W.T.; Carabelli A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock S.J. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021, 19, 409–424.
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 2021, 22,757–773 (2021). https://doi.org/10.1038/s41576-021-00408-x
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Agoramoorthy, G.; Lee S-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: lessons learned from major clinical studies. Front Pharmacol. 2021, 12, 2942. doi: 10.3389/fphar.2021.704205.
- Ayoub, M.A.; Crépieux, P.; Koglin, M.; Parmentier, M.; Pin, J.P.; Poupon, A.; Reiter, E.; Smit, M.; Steyaert, J.; Watier, H.; Wilkinson, T. Antibodies targeting G protein-coupled receptors: Recent advances and therapeutic challenges. MAbs. 2017, 9(5), 735-741.
- Butler DC, McLear JA, Messer A. Engineered antibody therapies to counteract mutant huntingtin and related toxic intracellular proteins. Prog Neurobiol. 2012, 97(2), 190-204.
- Škrlj, N.; Dolinar M. New engineered antibodies against prions. Bioengineered 2014, 5(1), 10-4.
- Angelini, A.: Miyabe, Y.: Newsted, D.: Kwan, B.H.; Miyabe, C.; Kelly, R.L.; Jamy, M.N.; Luster, A.D.; Wittrup, K.D. Directed evolution of broadly crossreactive chemokine-blocking antibodies efficacious in arthritis. Nat. Commun. 2018, 9(1), 1461.
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. scFv antibody: principles and clinical application. Clin. Dev. Immunol. 2012, 980250.
- Westlund, K.N.; Montera, M.A.; Goins, A.E.; Alles, S.R.A.; Afaghpour-Becklund, M.; Bartel, R.; Durvasula, R.; Kunamneni, A. Single-chain Fragment variable antibody targeting cholecystokinin-B receptor for pain reduction. Neurobiol. Pain. 2021 Jul 15;10:100067. doi: 10.1016/j.ynpai.2021.100067.
- Haviv, Y.; Zadik, Y.; Sharav, Y.; Benoliel, R. Painful Traumatic Trigeminal Neuropathy: An Open Study on the Pharmacotherapeutic Response to Stepped Treatment. J. Oral Facial Pain Headache 2014, 28, 52–60.
- Baad-Hansen, L.; Benoliel, R. Neuropathic Orofacial Pain: Facts and Fiction. Cephalalgia Int. J. Headache 2017, 37, 670–679.
- Westlund, K.N.; Montera, M.A.; Goins, A.E.; Alles, S.R.A.; Suri, N.; McIlwrath, S.L.; Bartel, R.; Durvasula, R.V.; Kunamneni, A. Single-Dose P2 X4R Single-Chain Fragment Variable Antibody Permanently Reverses Chronic Pain in Male Mice. Int. J. Mol. Sci.2021, 22, 13612. https://doi.org/10.3390/ijms222413612
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. (2015). Different immune cells mediate mechanical pain hypersensitivity in male and female mice. NatNeurosci2015, 18, 1081–1083.
This entry is adapted from the peer-reviewed paper 10.3390/antib9030028
