The human microbiome is a key factor in many malignancies, having the ability to alter host metabolism and immune responses and participate in tumorigenesis. Gut microbes have an influence on physiological functions of the healthy pancreas and are themselves controlled by pancreatic secretions. An altered oral microbiota may colonize the pancreas and cause local inflammation by the action of its metabolites, which may lead to carcinogenesis. The mechanisms behind dysbiosis and pancreatic cancer (PC) development are not completely clear. An altered microbiota may induce oncogenomic changes, or, on the other hand, cancer mutations may have an impact on microbiota composition. Altered microbiota can also influence drug efficacy in PC chemo- and immunotherapies. Possible future scenarios are the intentional manipulation of the gut microbiota in combination with therapy or the utilization of microbial profiles for the noninvasive screening and monitoring of PC.
- Pancreatic
- Cancer
- Microbiota
- Dysbiosis
- Oncogenomics
- Drug Response
- Bacterial Metabolites
- Inflammation
1. The Microbiome in Pancreatic Health
2. Microbiota Alterations in Pancreatic Cancer (PC)
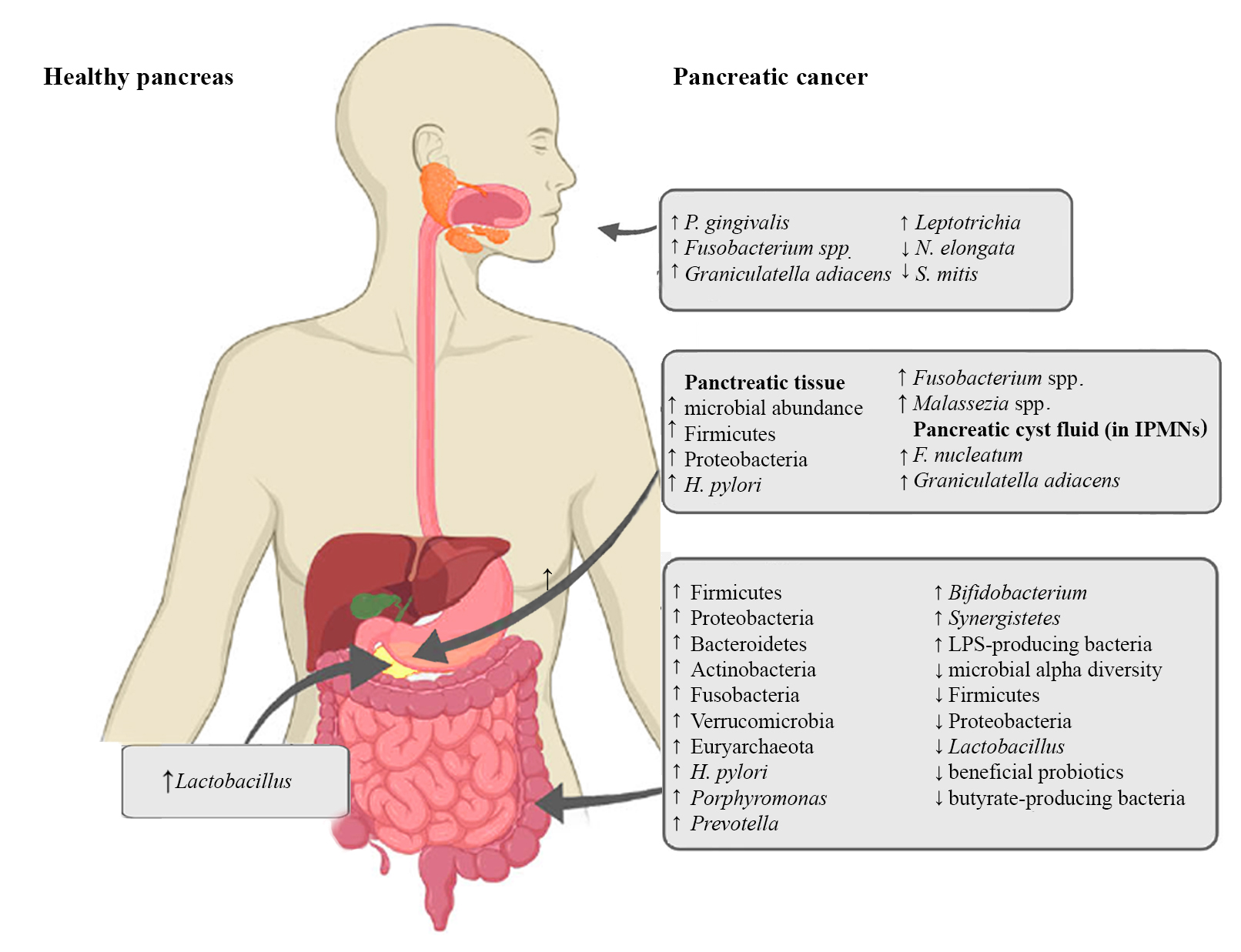
Figure 1. Microbiota alterations in PC
2.1. Oral Microbiota and PC
2.2. Pancreatic Microbiota and PC
2.3. The Intestinal Microbiota and PC
3. Microbiota in Pancreatic Inflammation, Oncogenesis and Tumor Immunity
4. The Microbiota and Drug Response in PC
This entry is adapted from the peer-reviewed paper 10.3390/ijms222312978
