Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Dendritic cells play a fundamental role in the antitumor immunity cycle, and the loss of their antigen-presenting function is a recognized mechanism of tumor evasion. Recently it demonstrated the effect of exosomes extracted from serum of patients with acute myeloid leukemia as important inducers of dendritic cell immunotolerance, adding evidence on the important role of intercellular communication by nanoparticles on antitumor responses.
- dendritic cells
- Immunity
- exosomes
1. Background
In recent years, there has been a growing interest in the role of non-neoplastic cells infiltrating the tumor environment, such as mesenchymal cells, endothelial cells, and immune system components. It is becoming increasingly evident that the biology of tumors, including their proliferation, invasion, and spread capabilities, depend largely on the support or repression of these other cell types, especially the cells of the immune system [1].
Tumor cells use various mechanisms to escape the immune system that may directly impact the prognosis of cancer patients and their response to currently available therapies. These actions include features such as attracting immunosuppressive cell populations to infiltrate the tumor microenvironment, as well as modulating normal immune cells to a more permissive and tolerant phenotype polarized for tumor growth and spread [2]. Furthermore, recent studies have shown the possibility of altering tumor growth and preventing its escape mechanisms by blocking or eliminating these dysfunctional immune cells, or by reprogramming their functions to a cytotoxic state [3].
2. Dendritic Cells Functionality in Intratumoral Immune Infiltrate
The discovery of dendritic cells in 1972 was considered a major milestone in understanding the functioning of the immune system and, later, in grasping the immunology of tumors [4]. Their specialized capabilities for antigen capture, processing, and presentation have been progressively described and can be seen in detail in Figure 1. Dendritic cells present a wide tissue distribution, acting as a surveillance system that connects the innate and adaptive immune systems. They are generated through bone marrow precursors and are classified into four general groups: conventional DCs (cDC), plasmocytoid DCs (pDC), monocyte derived DCs, and Langerhans cells. cDCs are further classified according to their tissue location, surface markers, and more recently by the expression of specific transcription factors as well [5].
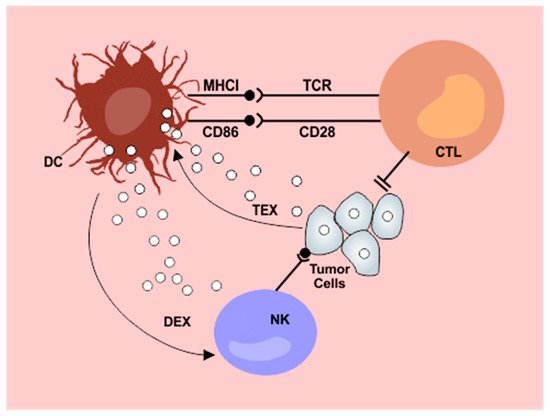
Figure 1. Interplay between dendritic cells and exosomes in the antitumor immunity cycle. Tumor derived exosomes (TEX) are internalized by dendritic cells (DC) resulting in impaired lymphocyte activation. Exosomes released from dendritic cells after contact with tumor antigens (DEX) potentiate NK cytotoxicity. DC: dendritic cells; NK: natural killer cells; CTL: cytotoxic T lymphocytes; DEX: dendritic cell derived exosomes; TEX: tumor derived exosomes; MHCI: major histocompatibility complex I. From: Cells 2019, 8(12), 1648
The elemental function of DCs is to prime and activate naive T cells for an adaptive immune response. In their immature form, they are avidly capable of antigen capture and are characterized by low expression of major histocompatibility complex (MHC) molecules and co-stimulatory molecules (such as CD80 and CD86) [6]. After recognition of molecular patterns associated with pathogens or other antigenic signals (including the presence of tumor cells), DCs undergo a maturation process with increased expression of MHC and co-stimulators on their surface, as well as releasing cytokines, which are essential for T lymphocyte activation [7][8].
In most tumors, the onset of the T cell-mediated cytotoxic immune response also begins with the presentation of disease-related antigens by the dendritic cells to the cytotoxic CD8+ and helper CD4+ T lymphocytes through molecules of the MHC class I and II, respectively. Following stimulation, naïve CD4+ T cells can be polarized into type 1 helper T cells (TH1), which in turn support the generation and proliferation of antigen-specific cytotoxic T lymphocytes. These lymphocytes (through their specific T cell receptor) are capable of recognizing tumor cells that exhibit the specific antigens to which they had been stimulated. Then follows the cytotoxic attack, for which these lymphocytes have different possible tools, such as cell death-associated receptor ligands (such as FasL) or cytolytic proteins released from intracytoplasmic granules (such as granzyme B and perforin) [9][10].
However, tumor clones may develop abilities to escape this defense system and proliferate leading to a new manifest disease or relapse. Tumor evasion can occur by several mechanisms, such as decreased immune recognition through the tumor cells’ production of antigens with lower immunogenicity, reduced expression of MHC molecules leading to lower antigen presentation capacity, and failure to activate effector cytotoxic cells. In addition, tumor cells can stimulate the development of an immunosuppressive tumor microenvironment through cytokine release and proliferation of regulatory T lymphocytes (Tregs) [11].
Precisely because of their inherent plasticity, DCs can become prone to the effects of the immunosuppressive tumor microenvironment: tumor cells can suppress the functions of DCs by polarizing them into an immunotolerant phenotype or by recruiting precursors of immunosuppressive DCs. Several mechanisms have been described to explain these changes, including the effect of various cytokines, such as IL-6 and IL-10, growth factors, and metabolic and oxidative changes [12][13]. More recently, the role of tumor-derived microvesicles has also begun to be unraveled in this process.
3. Tumor Immune Evasion: Role of Exosomes
Exosomes are small vesicles measuring approximately 30 to 150 nm in size secreted by different cell types, which have recently received great attention for their possible role as biomarkers in various pathological conditions [14]. Despite having been previously cited as mere cell debris, recent studies have shown an active role of exosomes in intercellular communication by transporting proteins, RNA, and microRNAs that can significantly alter the function of target cells [15][16]. The exosomes are now known to correspond to intraluminal vesicles of endosomal multivesicular bodies (MVBs), formed after endosome invagination and released into the extracellular space by fusion of the MVBs with the plasma membrane. Due to their cellular origin, these particles contain endosomal pathway-specific marker proteins such as tetraspanins (CD63, CD9, and CD81) and heat shock proteins (HSP70) [17].
The exosomes were first visualized in medium collected from reticulocyte cultures [18]; since then, several cell types have been identified as exosome sources, such as hematopoietic cells, epithelial cells, neurons, and adipocytes among others [19]. Initially, exosomes were suggested to play the role of removing molecules unnecessary for cellular metabolism that were only partially degraded by the lysosomal system [20]. However, as investigations progress, their functions appear to be considerably more complex: platelets secrete coagulation-regulating exosomes [21], extracellular vesicles of cardiac progenitors are capable of inhibiting cardiomyocyte apoptosis following myocardial infarction [22], and astrocyte-derived exosomes decrease neuronal damage caused by hypoxia through in vivo autophagy regulation [23].
It is clear from these observations that since exosomes carry particular profiles of proteins, RNA, and microRNAS but recap the internal content of their source cells, these nanoparticles appear to undergo a process of “selective packaging” as a way of refining and enhancing intercellular communication at distant sites and thus regulating important biological functions [24]. Exosomes are internalized by target cells through direct membrane fusion or endocytosis [25] and act mainly by regulating the expression of specific proteins. These effects can be accomplished through the direct transport of mRNAs to be translated or the delivery of microRNAs that lead to transcriptional repression and consequent genetic silencing [26][27].
Importantly, exosomes as molecular messengers have the potential to modulate several pathological scenarios, such as the maintenance of tumor microenvironments. This is accomplished through different biological processes, mainly those involved in immune responses and that include, for instance, signal transduction and antigen presentation [28][29][30]. In fact, there is growing evidence about the modulation of immune cells functions by exosomes, which can be particularly observed in the case of tumor-derived exosomes. Tumor cells can secrete exosomes capable of attenuating the responses of lymphocytes, macrophages, NK cells, and DCs, as well as promoting the expansion of myeloid-derived suppressor cells (MDSCs), a heterogeneous group of immature myeloid cells involved in states of immunosuppression [27][31].
However, some studies, in contrast, have pointed to a possible role of exosomes in antitumor immunovigilance as carriers of tumor antigens to be loaded into dendritic cells. Wolfers et al. showed that exosomes derived from solid tumors such as colon and breast cancer, when delivered to dendritic cells, lead to the activation of T-cell-mediated immune responses culminating in tumor rejection. Interestingly, there was cross-protection among the various tumors evaluated, pointing to the possibility that these exosomes carry common tumor antigens, which would be easily presented by MHC-I molecules in dendritic cells [32]. A similar approach was tested using exosomes derived from heat-stressed carcinoembryonic antigen (CEA) positive tumor cells. These particles induced DC maturation that culminated in cytotoxic lymphocytic responses and reduced tumor burden [33].
In our laboratory, we initially speculated that exosomes purified from serum of patients with hematologic malignancies could also be useful as an antigenic pulse in the development of new forms of immunotherapy. This seemed promising considering previous results with solid cancers and the fact that exosomes are released containing particular contents of proteins, RNAs, and microRNAs that recap the internal content of the maternal cells, and could thus contain specific antigenic material for the priming of dendritic cells. To evaluate this hypothesis, exosomes from serum of patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) were purified and used as an antigenic source for DCs in co-cultures with lymphocytes and leukemic K562 cells. Surprisingly, our results demonstrated that incubation of DCs with patients’ exosomes decreased the lysis of target cells, probably corresponding to an immune tumor evasion mechanism in vivo [34].
In fact, because they are responsible for T lymphocyte activation, DCs also play an important role in the sensitive balance between immune response and tolerance. Previous studies have shown that mature DCs can limit effector T-cell responses and promote immune tolerance in response to different signaling molecules such as IL-27 and IL-10 [35][36]. In the case of cancer patients, circulating exosomes could possibly be generated in the tumor microenvironment, also containing immunosuppressive molecules, constituting an effective mechanism for the paracrine induction of tolerance and therefore tumor escape.
Our group also demonstrated that these DCs stimulated with exosomes from AML patients, despite not altering lymphocyte proliferation rates, led to a marked decrease in INF-γ production by these effector cells, and INF-γ levels were inversely related to CD86 expression in DCs. Therefore, we can speculate that in AML, the exosome-induced suppression of cytotoxicity may, at least in part, be the result of dysregulation in co-stimulatory molecules in DCs, such as CD86, leading to decreased activation of lymphocytes with impaired INF-γ production [34].
Interestingly, a study with a murine model of AML had previously demonstrated the effectiveness of using exosomes as a pulse of DCs [37]. One possible explanation for this discrepancy in relation to human patients may be that the tumor in mice was not autologous, but tumor cells had been injected into these animals. This approach may not necessarily mimic the systemic immunosuppressive environment involved in AML in humans, which may explain why promising preclinical results have not been reproduced in human trials.
Furthermore, our results are in line with the latest studies published on this topic, consolidating the idea that exosomes participate in the induction of immunotolerance in AML. Hong et al. evaluated AML patients treated with NK cell infusion and could observe the effect of patients’ exosomes present in pretreatment samples. These authors noted that there was a decrease in the cytotoxicity of these NK cells when they were incubated along with the serum containing exosomes that was extracted from patients prior to treatment [38]. Moreover, specifically in relation to dendritic cells, a recent study with prostate cancer patients demonstrated that after incubation of DCs with patients’ exosomes, there was also a significant decrease in the release of inflammatory cytokines and less activation of INF-producing CD8+ lymphocytes [39]. Recent studies have also demonstrated the immunosuppressive potential of extracellular vesicles in gliomas [40], and melanoma-derived exosomes have been shown to inhibit the differentiation of monocytic precursors in dendritic cells, leading to increased TGF-β production and suppression of lymphocyte proliferation [41].
This entry is adapted from the peer-reviewed paper 10.3390/cells8121648
References
- Kerkar, S.P.; Restifo, N.P. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 2012, 72, 3125–3130.
- Zitvogel, L.; Kepp, O.; Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160.
- Liu, Y.; Cao, X. Immunosuppressive cells in tumor immune escape and metastasis. J. Mol. Med. 2016, 94, 509–522.
- Steinman, R.M.; Cohn, Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. J. Exp. Med. 1973, 137, 1142–1162.
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21.
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252.
- Sparwasser, T.; Wong, P.; Wu, S.; Ko, K.; Lang, R.A.; Zavala, F.; Jung, S.; Sano, G.-I.; Vuthoori, S.; De los Santos, K.; et al. In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity 2004, 17, 211–220.
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell 2014, 26, 638–652.
- Van Acker, H.H.; Versteven, M.; Lichtenegger, F.S.; Roex, G.; Campillo-Davo, D.; Lion, E.; Subklewe, M.; Van Tendeloo, V.F.; Berneman, Z.N.; Anguille, S. Dendritic Cell-Based Immunotherapy of Acute Myeloid Leukemia. J. Clin. Med. 2019, 8, 579.
- Barrett, A.J.; Le Blanc, K. Immunotherapy prospects for acute myeloid leukaemia. Clin. Exp. Immunol. 2010, 161, 223–232.
- Aldarouish, M.; Wang, C. Trends and advances in tumor immunology and lung cancer immunotherapy. J. Exp. Clin. Cancer Res. 2016, 35, 1–13.
- Tang, M.; Diao, J.; Cattral, M.S. Molecular mechanisms involved in dendritic cell dysfunction in cancer. Cell. Mol. Life Sci. 2017, 74, 761–776.
- Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat. Rev. Immunol. 2004, 4, 941–952.
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The future of biomarkers in medicine. Biomark. Med. 2013, 7, 769–778.
- Fleming, A.; Sampey, G.; Chung, M.C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 107–118.
- Katsuda, T.; Ikeda, S.; Yoshioka, Y.; Kosaka, N.; Kawamata, M.; Ochiya, T. Physiological and pathological relevance of secretory microRNAs and a perspective on their clinical application. Biol. Chem. 2014, 395, 365–373.
- D’Souza-Schorey, C.; Schorey, J.S. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018, 62, 125–133.
- Pan, B.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron Microscopic Evidence for Externalization of the Transferrin Receptor in Vesicular Form in Sheep Reticulocytes. J. Cell Biol. 1985, 101, 942–948.
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321.
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978.
- Heijnen, B.H.F.G.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles. Blood 1991, 94, 3791–3799.
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles fromhuman cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function aftermyocardial infarction. Cardiovasc. Res. 2014, 103, 530–541.
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 11474.
- Emanueli, C.; Shearn, A.I.U.; Angelini, G.D.; Sahoo, S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul. Pharmacol. 2015, 71, 24–30.
- Mulcahy, L.A.; Pink, R.C.; Raul, D.; Carter, F.; Ann, L.; Pink, R.C.; Raul, D.; Carter, F.; Mulcahy, L.A.; Pink, R.C.; et al. Routes and mechanisms of extracellular vesicle uptake. J. Extracell Vesicles 2014, 3078.
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641.
- Lu, J.; Wu, J.; Tian, J.; Wang, S. Role of T cell-derived exosomes in immunoregulation. Immunol. Res. 2018, 66, 313–322.
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteomics 2015, 15, 260–271.
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282.
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81.
- Graner, M.W. Tumor-derived exosomes, microRNAs, and cancer immune suppression. Semin. Immunopathol. 2018, 40, 505–515.
- Unit, I. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 38, 297–303.
- Dai, S.; Wan, T.; Wang, B.; Zhou, X.; Xiu, F.; Chen, T. Cancer Therapy: Preclinical More Efficient Induction of HLA-A * 0201-Restricted and Carcinoembryonic Antigen (CEA) ^ Specific CTL Response by Immunization with Exosomes Prepared from Heat-Stressed CEA-Positive T umor Cells. Clin. Cancer Res. 2005, 11, 7554–7564.
- Benites, B.D.; da Silva Santos Duarte, A.; Longhini, A.L.F.; Santos, I.; Alvarez, M.C.; de Morais Ribeiro, L.N.; Paula, E.; Saad, S.T.O. Exosomes in the serum of Acute Myeloid Leukemia patients induce dendritic cell tolerance: Implications for immunotherapy. Vaccine 2019, 37, 1377–1383.
- Mascanfroni, I.D.; Yeste, A.; Vieira, S.M.; Burns, E.J.; Patel, B.; Sloma, I.; Wu, Y.; Mayo, L.; Ben-Hamo, R.; Efroni, S.; et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 2013, 14, 1054–1063.
- Bruchhage, K.L.; Heinrichs, S.; Wollenberg, B.; Pries, R. IL-10 in the microenvironment of HNSCC inhibits the CpG OdN-induced IFN-α secretion of PDCS. Oncol. Lett. 2018, 15, 3985–3990.
- Gu, X.; Erb, U.; Büchler, M.W.; Zöller, M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer 2015, 136, E74–E84.
- Hong, C.; Sharma, P.; Yerneni, S.S.; Simms, P.; Jackson, E.K.; Whiteside, T.L.; Boyiadzis, M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017, 31, 1–10.
- Salimu, J.; Webber, J.; Gurney, M.; Al-taei, S.; Clayton, A.; Salimu, J.; Webber, J.; Gurney, M.; Al-taei, S.; Clayton, A. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracell. Vesicles 2017, 6, 1368823.
- Hellwinkel, J.E.; Redzic, J.S.; Harland, T.A.; Gunaydin, D.; Anchordoquy, T.J.; Graner, M.W. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro-Oncol. 2016, 18, 497–506.
- Valenti, R.; Huber, V.; Filipazzi, P.; Pilla, L.; Sovena, G.; Villa, A.; Corbelli, A.; Fais, S.; Parmiani, G.; Rivoltini, L. Human Tumor-Released Microvesicles Promote the Differentiation of Myeloid Cells with Transforming Growth Factor- B—Mediated Suppressive Activity on T Lymphocytes. Cancer Res. 2006, 66, 9290–9299.
This entry is offline, you can click here to edit this entry!
