Fungi are widely distributed in the terrestrial environment, freshwater, and marine habitat. Only approximately 100,000 of these have been classified although there are about 5.1 million characteristic fungi all over the world. These eukaryotic microbes produce specialized metabolites and participate in a variety of ecological functions, such as quorum detection, chemical defense, allelopathy, and maintenance of symbiosis. Fungi therefore remain an important resource for the screening and discovery of biologically active natural products. Sesquiterpenoids are arguably the richest natural products from plants and micro-organisms. The rearrangement of the 15 high-ductility carbons gave rise to a large number of different skeletons. At the same time, abundant structural variations lead to a diversification of biological activity.
- sesquiterpenoids
- fungus
- structures
- structural diversity
- biological activity
- synthesis
1. Introduction
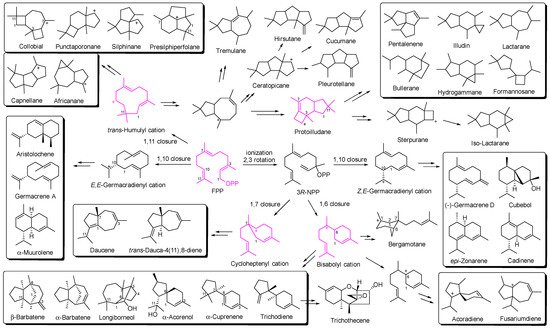
2. Composition and Bioactivities
2.1. Alliacane, Cadinene, Azulene, and Zierane
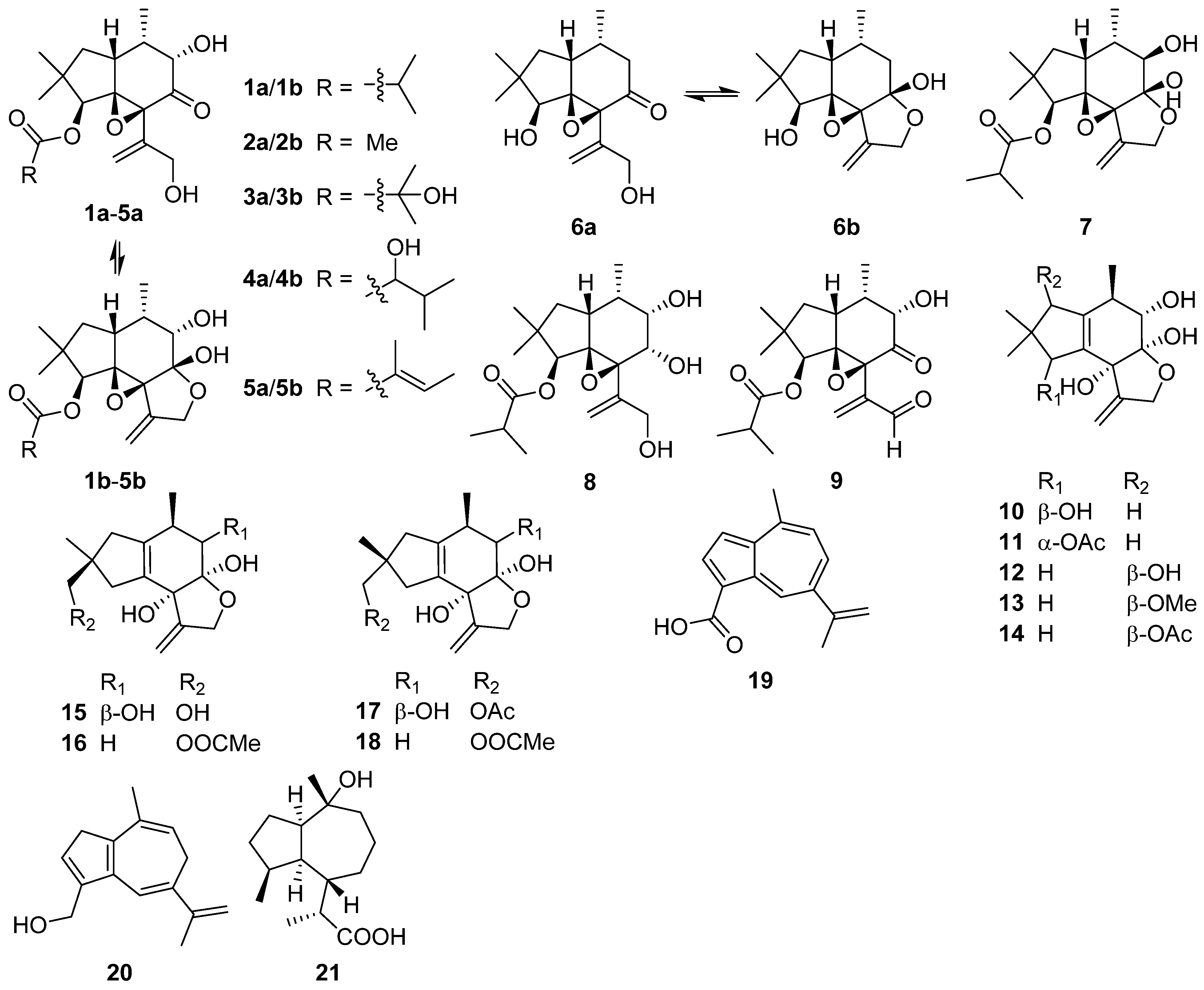
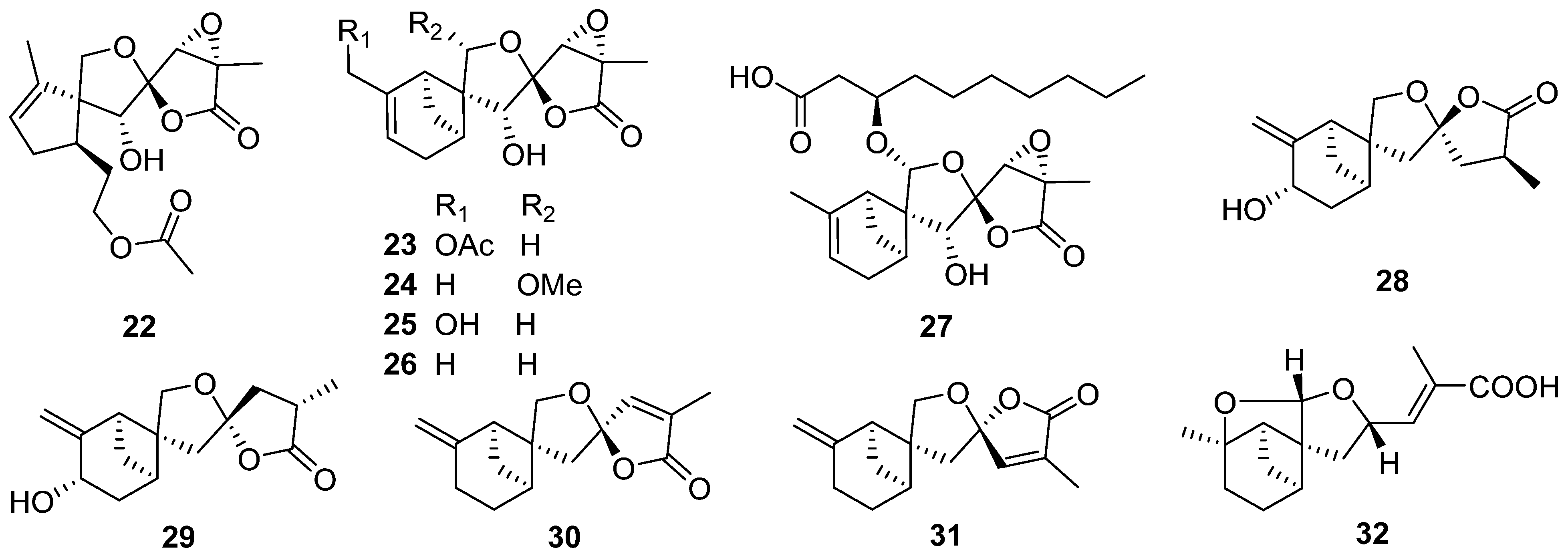
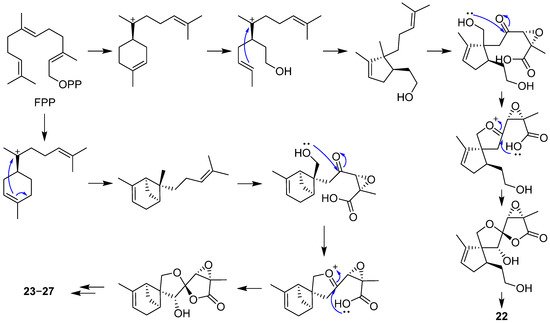
2.2. Bergamotane, Spiroaminal, and Spiroaxane
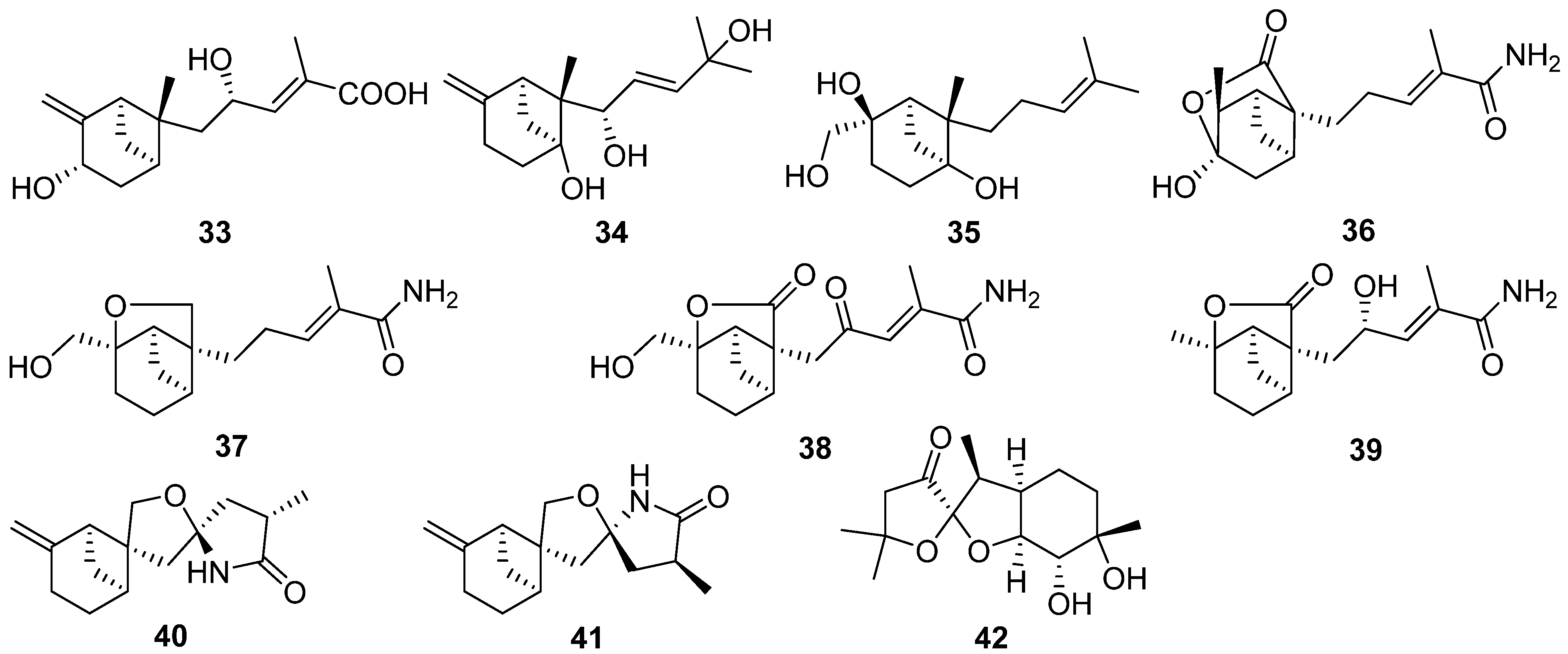
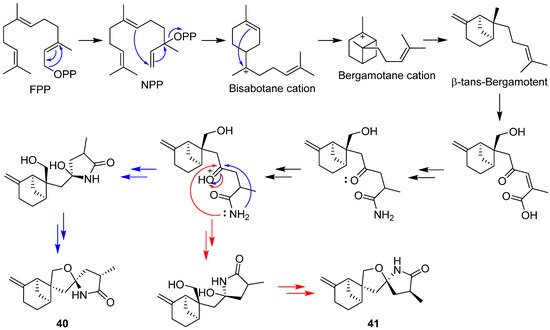
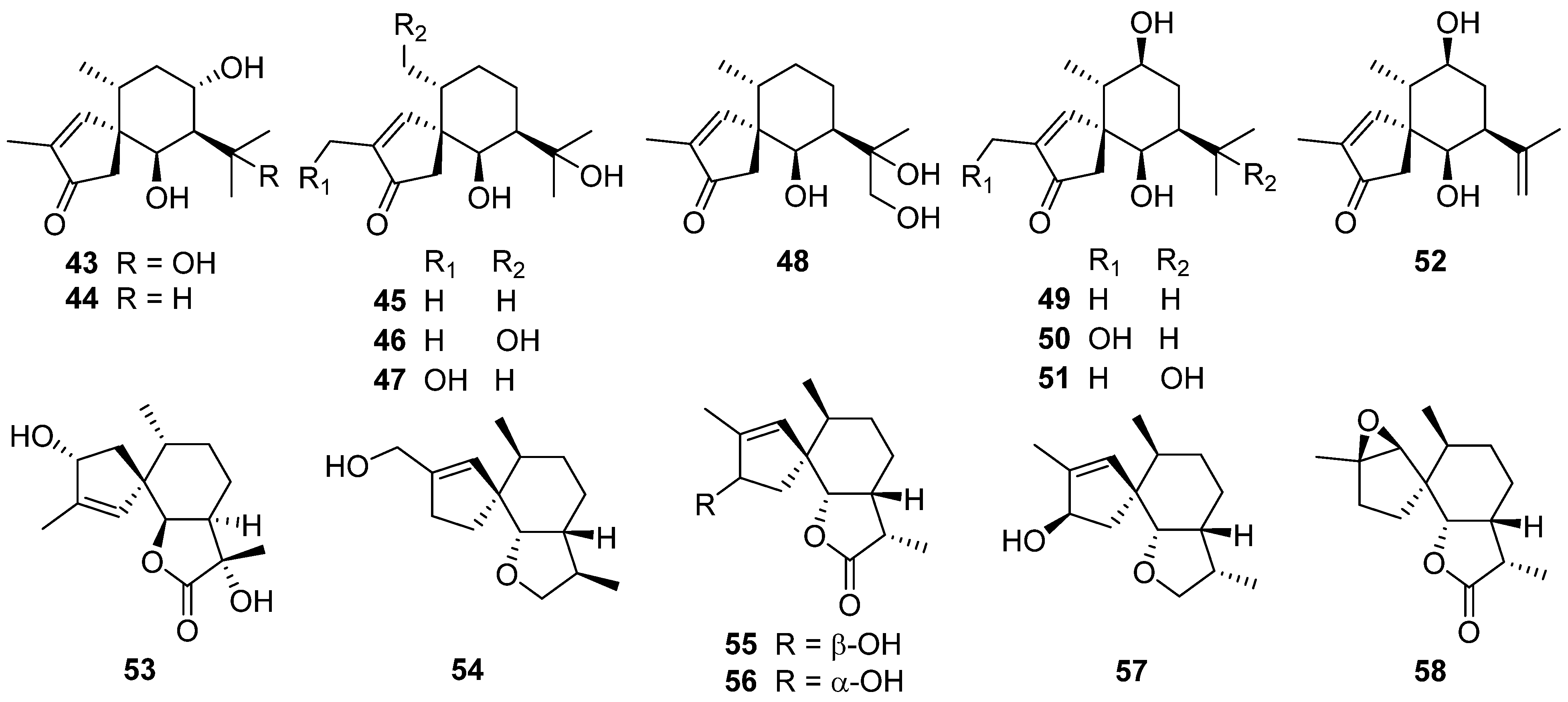
2.3. Carotane, Cyclonerane, Cyclofarnesane, and Longifolene
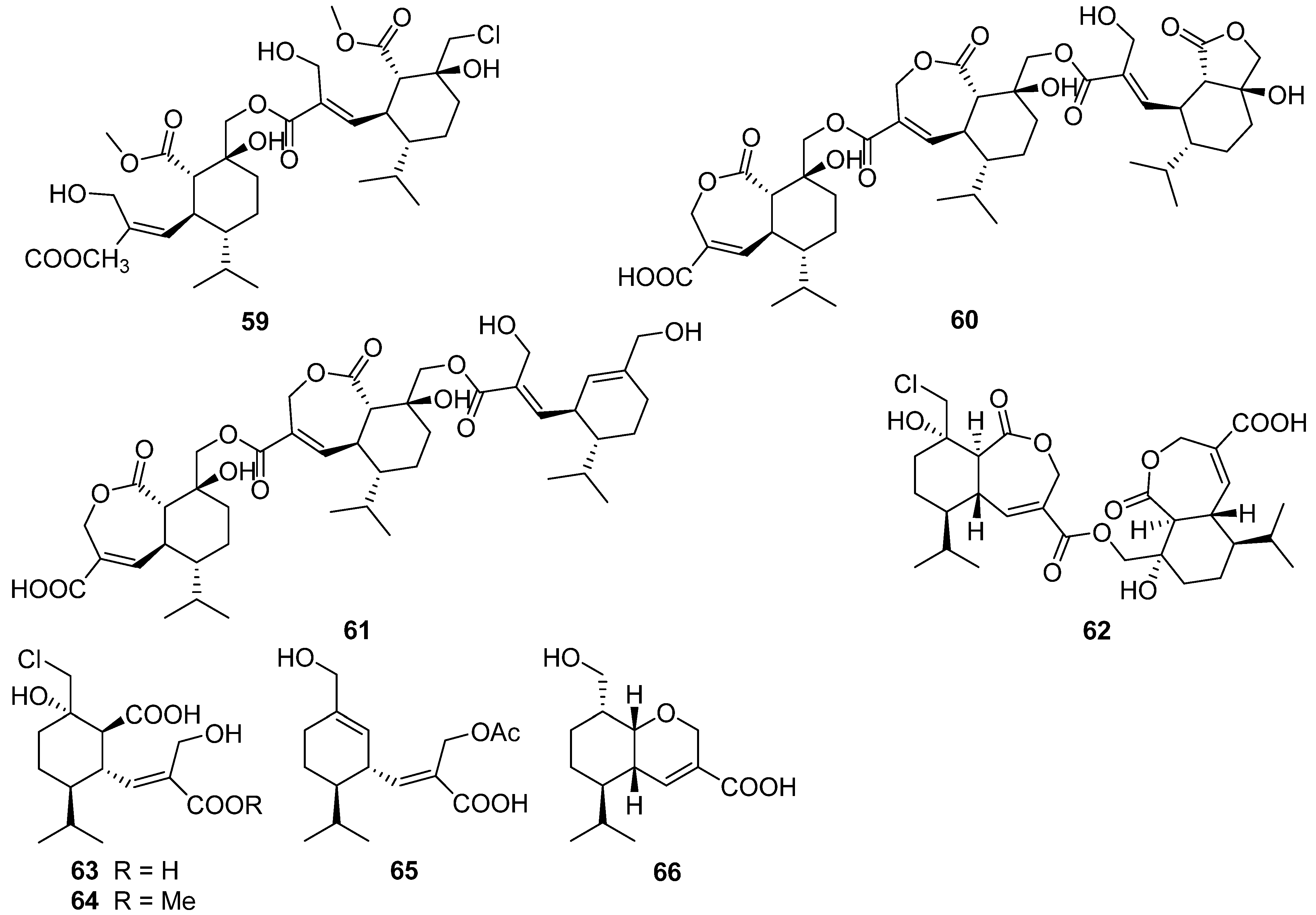
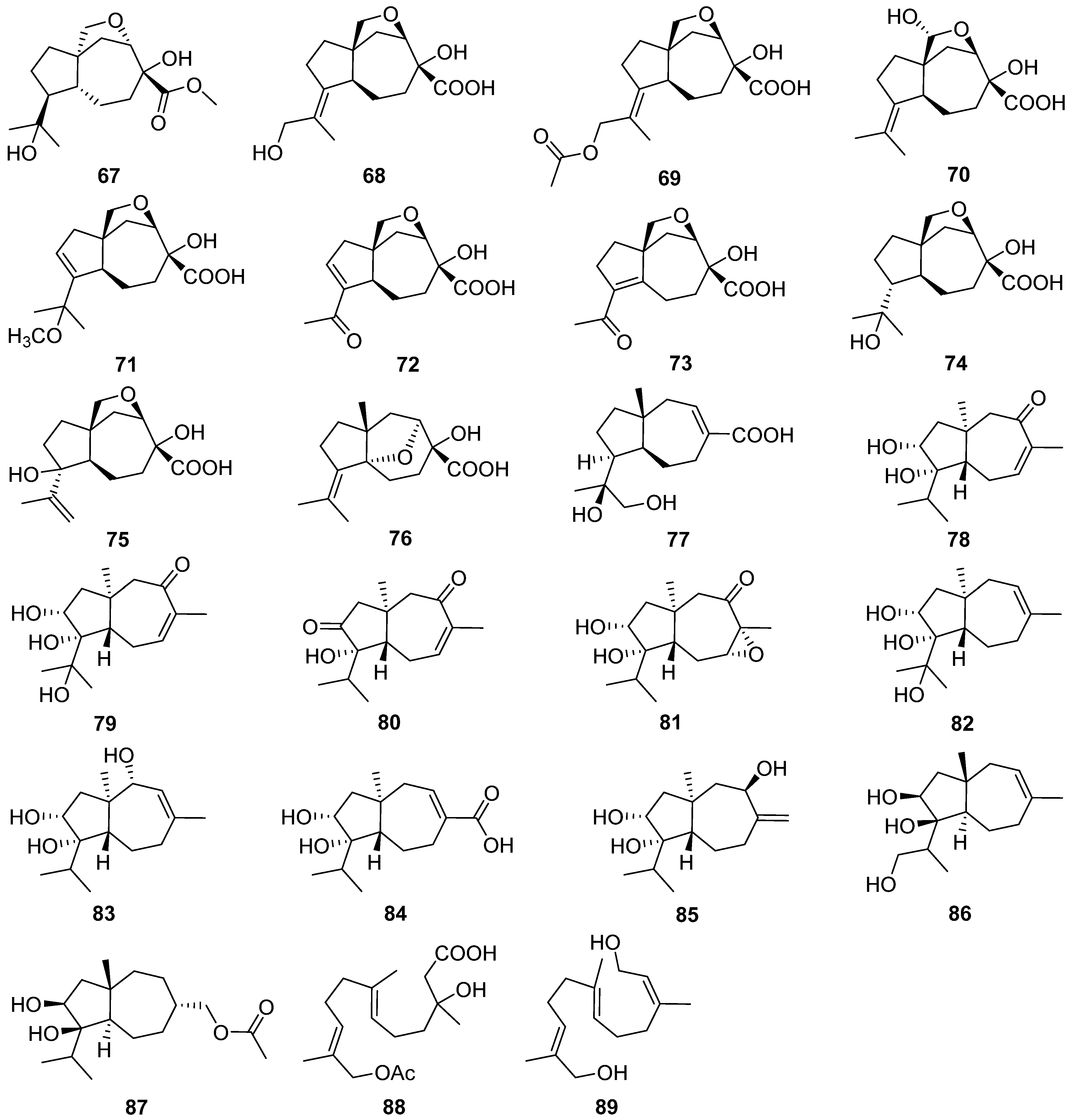
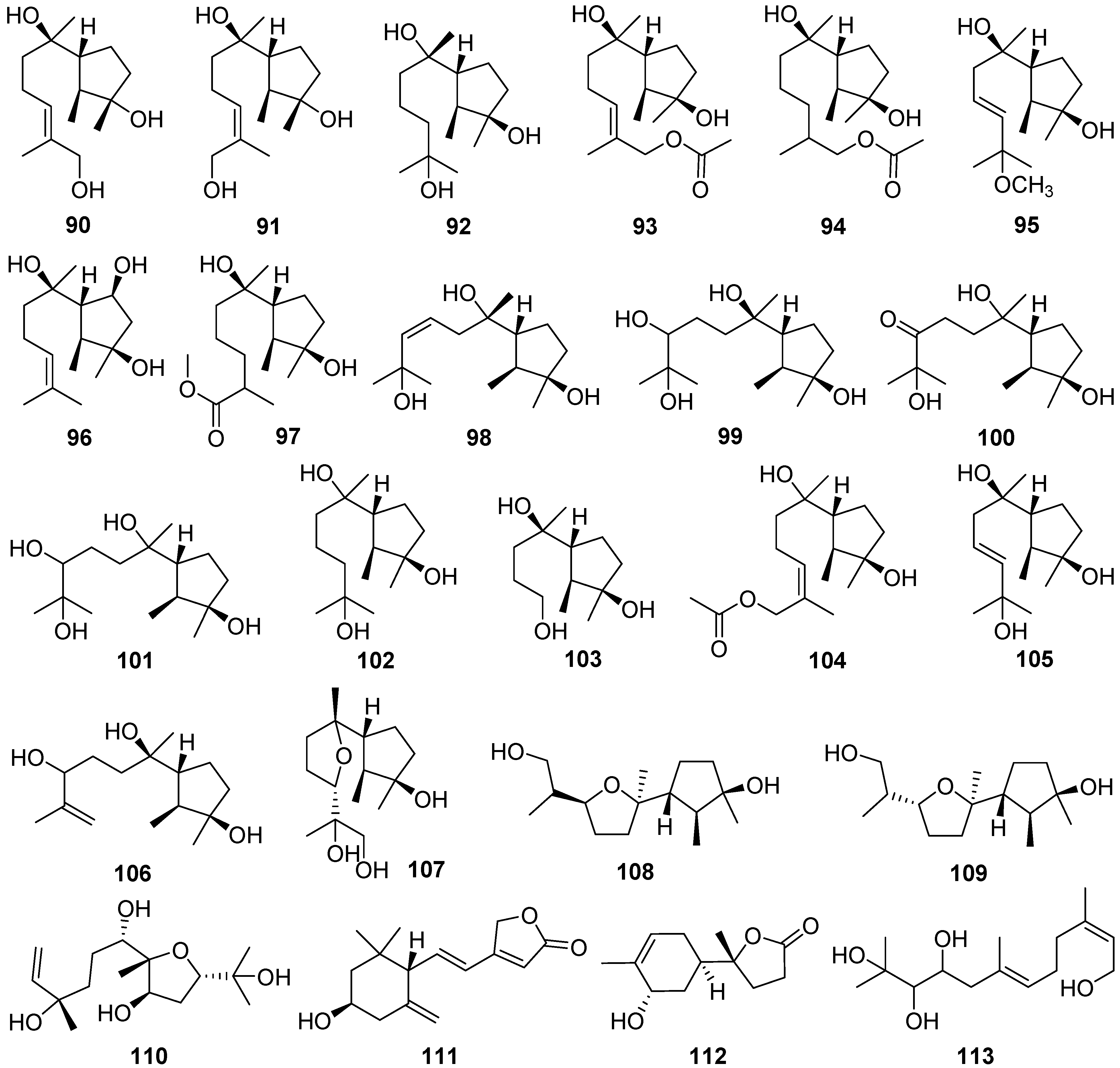
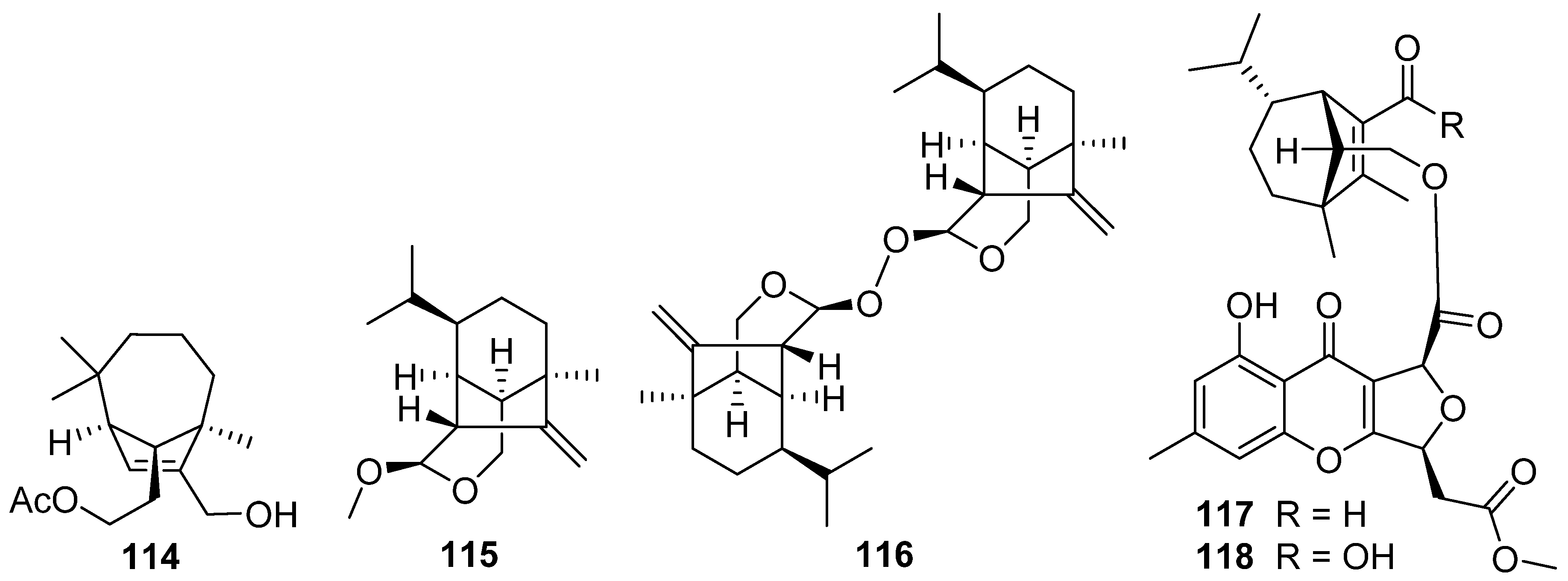
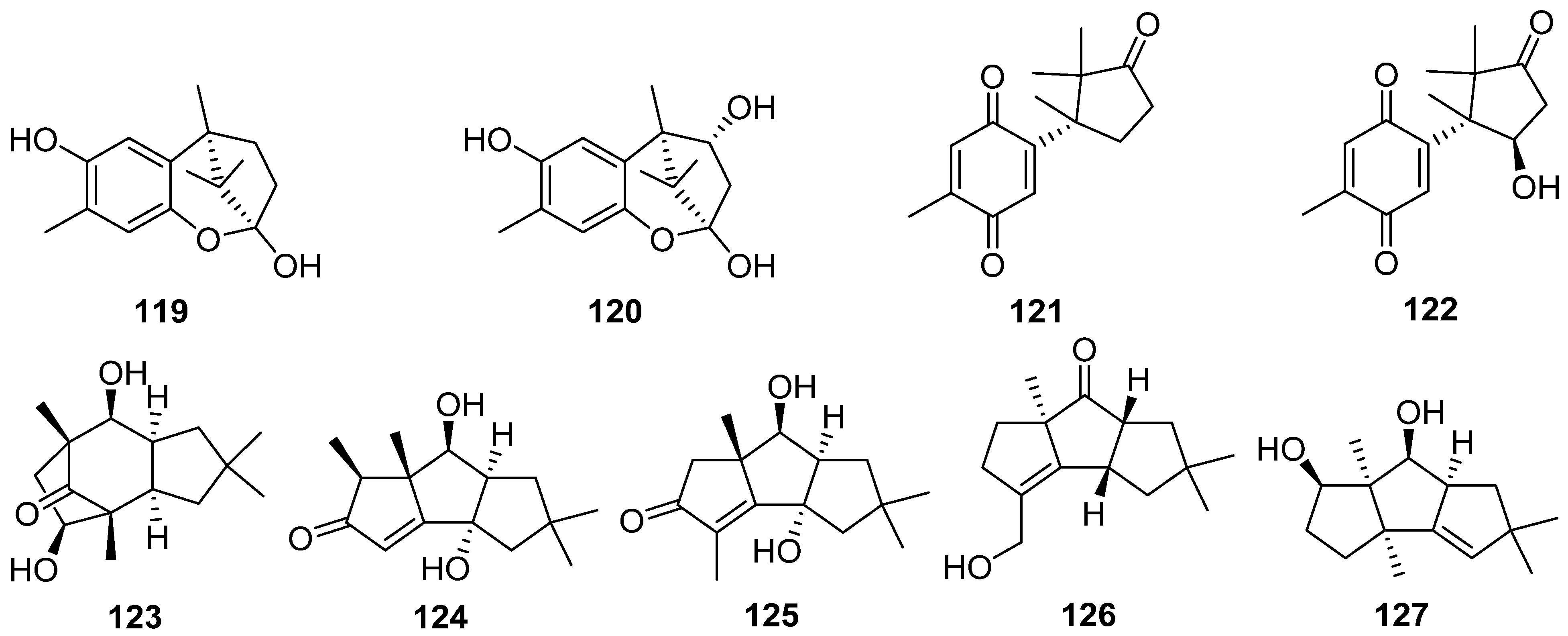
2.4. Cerapicane, Cucumane, Cuparene, Hirsutane, Isohirsutane, and Triquinane
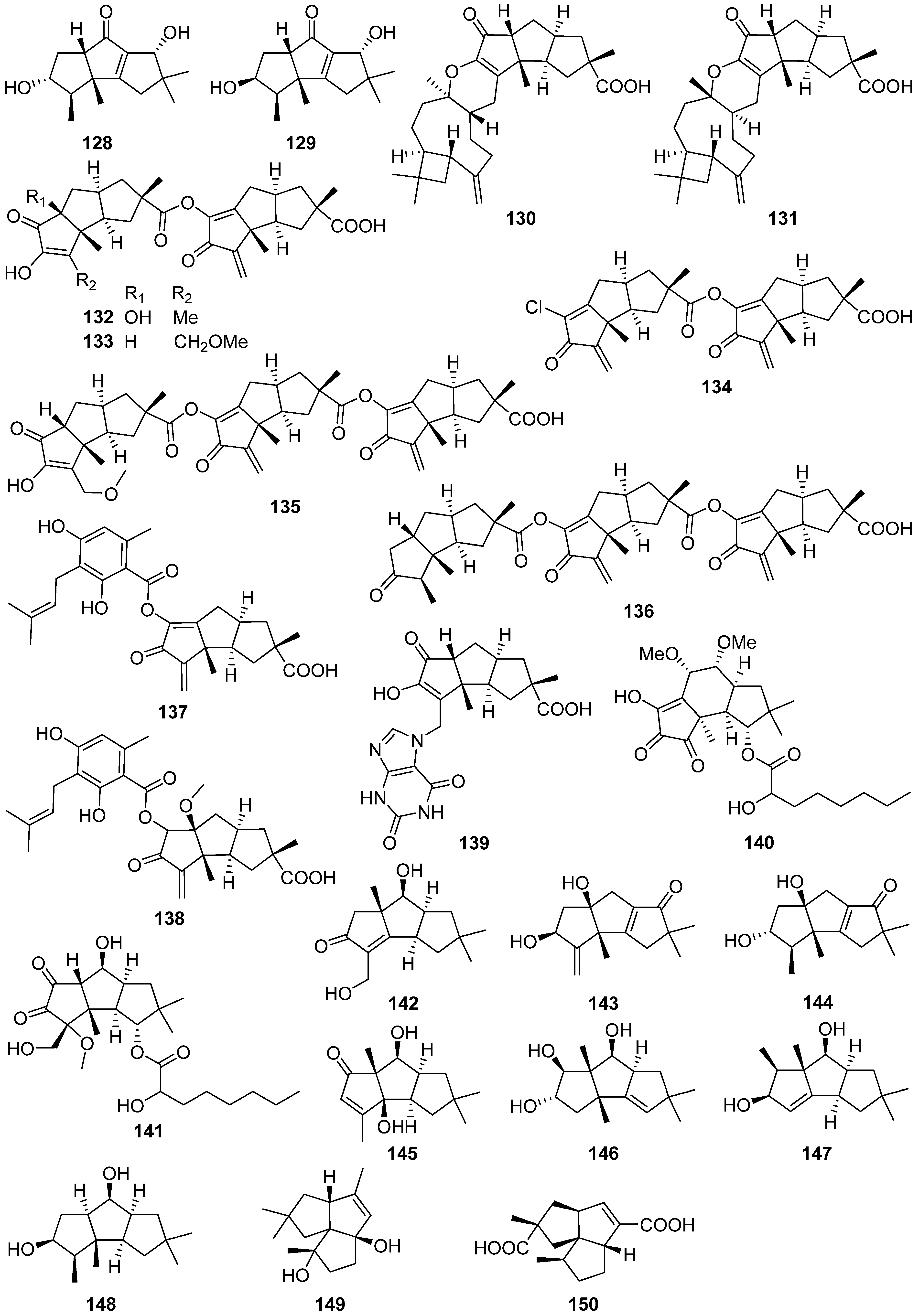
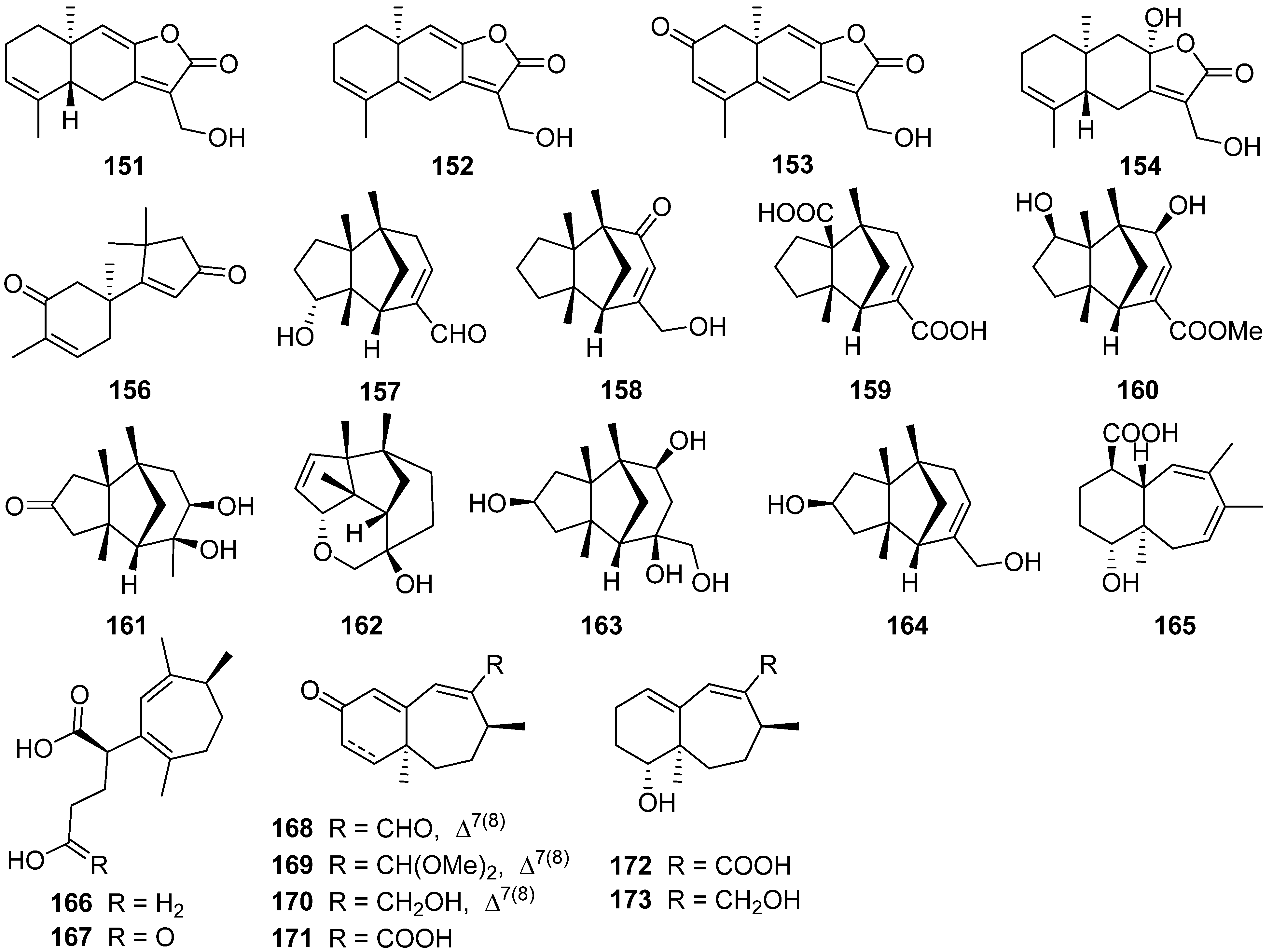
2.5. Eudesmanolide, Gymnomitrane, and Humulane
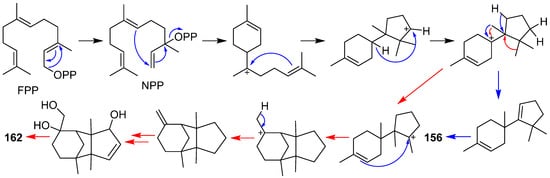
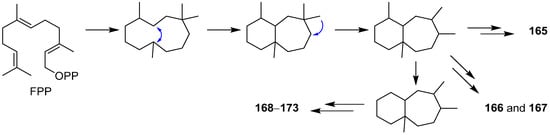
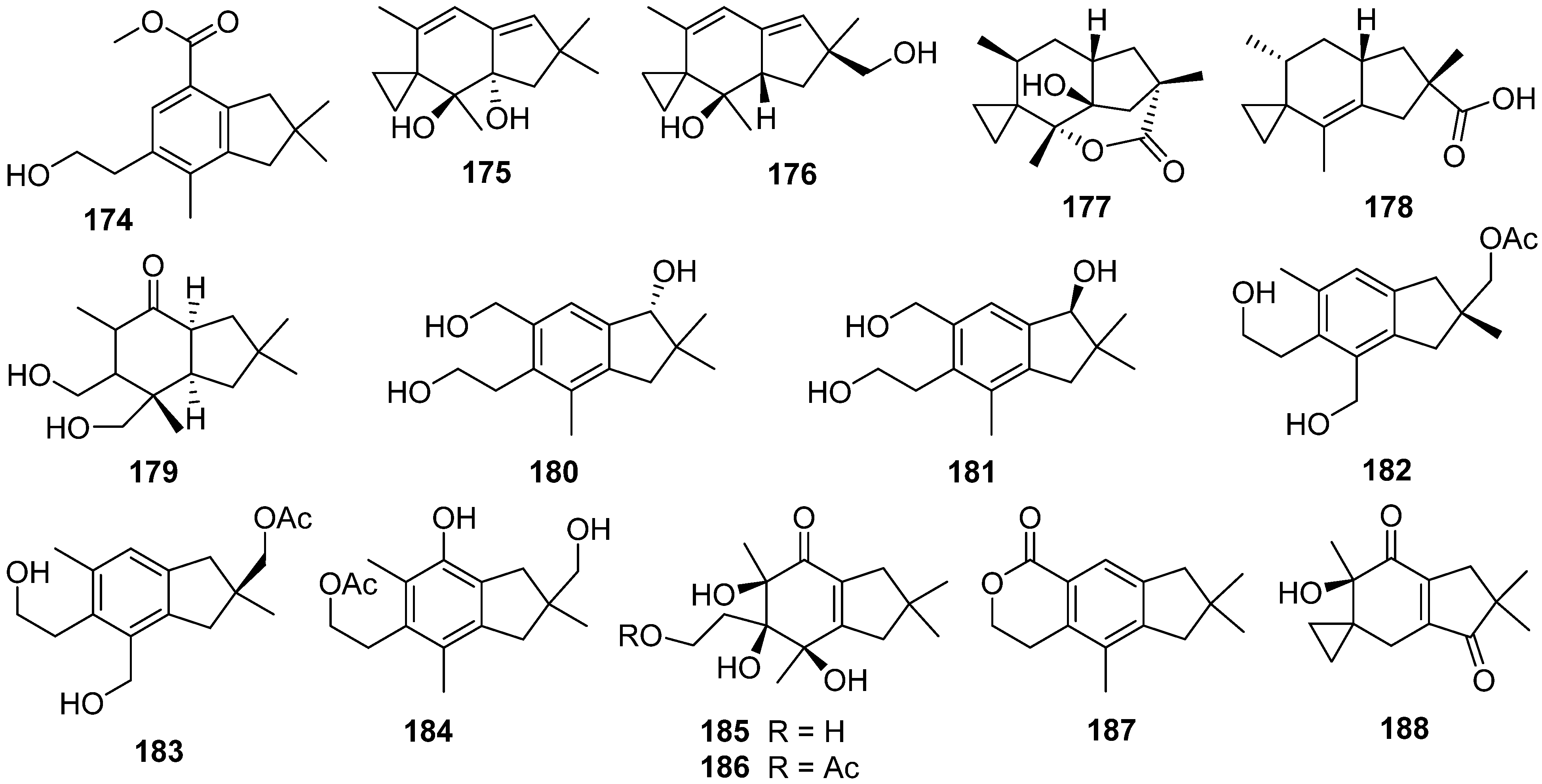
This entry is adapted from the peer-reviewed paper 10.3390/jof7121026
References
- Christianson, D.W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150.
- Minami, A.; Ozaki, T.; Liu, C.; Oikawa, H. Cyclopentane-forming di/sesterterpene synthases: Widely distributed enzymes in bacteria, fungi, and plants. Nat. Prod. Rep. 2018, 35, 1330–1346.
- Schmidt-Dannert, C. Biosynthesis of Terpenoid Natural Products in Fungi. Advances in Biochemical Engineering-Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: New York, NY, USA, 2015; Volume 148, pp. 19–61.
- Li, D.; Wang, K.W. Natural new sesquiterpenes: Structural diversity and bioactivity. Curr. Org. Chem. 2016, 20, 994–1042.
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 29, 1334–1366.
- Chen, H.P.; Liu, J.K. Secondary metabolites from higher fungi. Prog. Chem. Org. Nat. Prod. 2017, 106, 1–201.
- Gonzalez Del Val, A.; Platas, G.; Arenal, F.; Orihuela, J.C.; Garcia, M.; Hernandez, P.; Royo, I.; De Pedro, N.; Silver, L.L.; Young, K.; et al. Novel illudins from Coprinopsis episcopalis (syn. Coprinus episcopalis), and the distribution of illudin-like compounds among filamentous fungi. Mycol. Res. 2003, 107, 1201–1209.
- Alexandre, J.; Raymond, E.; Kaci, M.O.; Brain, E.C.; Lokiec, F.; Kahatt, C.; Faivre, S.; Yovine, A.; Goldwasser, F.; Smith, S.L.; et al. Phase I and pharmacokinetic study of irofulven administered weekly or biweekly in advanced solid tumor patients. Clin. Cancer Res. 2004, 10, 3377–3385.
- Tanasova, M.; Sturla, S.J. Chemistry and biology of acylfulvenes: Sesquiterpene-derived antitumor agents. Chem. Rev. 2012, 112, 3578–3610.
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of wheat and barley: A re-emerging disease of devastating impact. Plant Dis. 1997, 81, 1340–1348.
- Eriksen, G.S.; Pettersson, H. Toxicological evaluation of trichothecenes in animal feed. Anim. Feed Sci. Technol. 2004, 114, 205–239.
- Qinghua, W.; Vlastimil, D.; Kami, K.; Zonghui, Y. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660.
- Pascari, X.; Maul, R.; Kemmlein, S.; Marin, S.; Sanchis, V. The fate of several trichothecenes and zearalenone during roasting and enzymatic treatment of cereal flour applied in cereal-based infant food production. Food Control 2020, 114, 107245.
- Isaka, M.; Sappan, M.; Supothina, S.; Srichomthong, K.; Komwijit, S.; Boonpratuang, T. Alliacane sesquiterpenoids from submerged cultures of the basidiomycete Inonotus sp. BCC 22670. Phytochemistry 2017, 136, 175–181.
- Tao, Q.Q.; Ma, K.; Bao, L.; Wang, K.; Han, J.J.; Zhang, J.X.; Huang, C.Y.; Liu, H.W. New sesquiterpenoids from the edible mushroom Pleurotus cystidiosus and their inhibitory activity against alpha-glucosidase and PTP1B. Fitoterapia 2016, 111, 29–35.
- Tala, M.F.; Qin, J.C.; Ndongo, J.T.; Laatsch, H. New azulene-type sesquiterpenoids from the fruiting bodies of Lactarius deliciosus. Nat. Prod. Bioprospect. 2017, 7, 269–273.
- Liu, S.; Dai, H.F.; Heering, C.; Janiak, C.; Lin, W.H.; Liu, Z.; Proksch, P. Inducing new secondary metabolites through co-cultivation of the fungus Pestalotiopsis sp. with the bacterium Bacillus subtilis. Tetrahedron Lett. 2017, 58, 257–261.
- Cane, D.E. Enzymic formation of sesquiterpenes. Chem. Rev. 1990, 90, 1089–1103.
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264.
- Massias, M.; Rebuffat, S.; Molho, L.; Chiaroni, A.; Riche, C.; Bodo, B. Expansolides A and B: Tetracyclic sesquiterpene lactones from Penicillium expansum. J. Am. Chem. Soc. 1990, 112, 8112–8115.
- Oh, H.; Gloer, J.B.; Shearer, C.A. Massarinolins A-C: New bioactive sesquiterpenoids from the aquatic fungus Massarina tunicata. J. Nat. Prod. 1999, 62, 497–501.
- Che, Y.; Gloer, J.B.; Koster, B.; Malloch, D. Decipinin A and decipienolides A and B: New bioactive metabolites from the coprophilous fungus Podospora decipiens. J. Nat. Prod. 2002, 65, 916–919.
- MacíAs, F.A.; Varela, R.M.; Simonet, A.M.; Cutler, H.G.; Cutler, S.J.; Hill, R.A. Absolute configuration of bioactive expansolides A and B from Aspergillus fumigatus Fresenius. Tetrahedron Lett. 2003, 44, 941–943.
- Wang, Y.N.; Xia, G.Y.; Wang, L.Y.; Ge, G.B.; Zhang, H.W.; Zhang, J.F.; Wu, Y.Z.; Lin, S. Purpurolide A, 5/5/5 spirocyclic sesquiterpene lactone in nature from the endophytic fungus Penicillium purpurogenum. Org. Lett. 2018, 20, 7341–7344.
- Xia, G.Y.; Wang, L.Y.; Zhang, J.F.; Wu, Y.Z.; Ge, G.B.; Wang, Y.N.; Lin, P.C.; Lin, S. Three new polyoxygenated bergamotanes from the endophytic fungus Penicillium purpurogenum IMM 003 and their inhibitory activity against pancreatic lipase. Chin. J. Nat. Med. 2020, 18, 75–80.
- Ying, Y.M.; Fang, C.A.; Yao, F.Q.; Yu, Y.; Shen, Y.; Hou, Z.N.; Wang, Z.; Zhang, W.; Shan, W.G.; Zhan, Z.J. Bergamotane sesquiterpenes with α-glucosidase inhibitory activity from the plant pathogenic fungus Penicillium expansum. Chem. Biodivers. 2017, 14, e1600184.
- Zhao, Z.Z.; Zhao, K.; Chen, H.P.; Bai, X.; Zhang, L.; Liu, J.K. Terpenoids from the mushroom-associated fungus Montagnula donacina. Phytochemistry 2018, 147, 21–29.
- Wang, Y.; Li, D.H.; Li, Z.L.; Sun, Y.J.; Hua, H.M.; Liu, T.; Bai, J. Terpenoids from the marine-derived fungus Aspergillus fumigatus YK-7. Molecules 2016, 21, 31.
- Guo, Z.; Ren, F.X.; Che, Y.S.; Liu, G.; Liu, L. New bergamotane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Molecules 2015, 20, 14611–14620.
- Zhang, L.H.; Feng, B.M.; Chen, G.; Li, S.G.; Sun, Y.; Wu, H.H.; Bai, J.; Hua, H.M.; Wang, H.F.; Pei, Y.H. Sporulaminals A and B: A pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 2016, 6, 42361–42366.
- Tao, Q.Q.; Ma, K.; Bao, L.; Wang, K.; Han, J.J.; Wang, W.Z.; Zhang, J.X.; Huang, C.Y.; Liu, H.W. Sesquiterpenoids with PTP1B inhibitory activity and cytotoxicity from the edible mushroom Pleurotus citrinopileatus. Planta Med. 2016, 82, 639–644.
- Tao, Q.Q.; Ma, K.; Yang, Y.L.; Wang, K.; Chen, B.S.; Huang, Y.; Han, J.J.; Bao, L.; Liu, X.B.; Yang, Z.L.; et al. Bioactive sesquiterpenes from the edible mushroom Flammulina velutipes and their biosynthetic pathway confirmed by genome analysis and chemical evidence. J. Org. Chem. 2016, 81, 9867–9877.
- Chen, C.M.; Sun, W.G.; Liu, X.R.; Wei, M.S.; Liang, Y.; Wang, J.P.; Zhu, H.C.; Zhang, Y.H. Anti-inflammatory spiroaxane and drimane sesquiterpenoids from Talaromyces minioluteus (Penicillium minioluteum). Bioorg. Chem. 2019, 91, 103166.
- Yang, X.Y.; Niu, W.R.; Li, R.T.; Cui, X.M.; Liu, J.K. Two new sesquiterpenes from cultures of the higher fungus Pholiota nameko. Nat. Prod. Res. 2018, 33, 1992–1996.
- Wang, S.R.; Zhang, L.; Chen, H.P.; Li, Z.H.; Dong, Z.J.; Wei, K.; Liu, J.K. Four new spiroaxane sesquiterpenes and one new rosenonolactone derivative from cultures of Basidiomycete Trametes versicolor. Fitoterapia 2015, 105, 127–131.
- Hu, Z.B.; Tao, Y.W.; Tao, X.Y.; Su, Q.H.; Cai, J.C.; Qin, C.; Ding, W.J.; Li, C.Y. Sesquiterpenes with phytopathogenic fungi inhibitory activities from fungus Trichoderma virens from Litchi chinensis Sonn. J. Agric. Food Chem. 2019, 67, 10646–10652.
- Liu, S.; Zhao, Y.P.; Heering, C.; Janiak, C.; Muller, W.E.G.; Akone, S.H.; Liu, Z.; Proksch, P. Sesquiterpenoids from the endophytic fungus Rhinocladiella similis. J. Nat. Prod. 2019, 82, 1055–1062.
- Duan, R.T.; Yang, R.N.; Li, H.T.; Tang, L.H.; Liu, T.; Yang, Y.B.; Zhou, H.; Ding, Z.T. Peniterester, a carotane-type antibacterial sesquiterpene from an artificial mutant Penicillium sp. T2-M20. Fitoterapia 2020, 140, 104422.
- Xing, C.P.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Lin, W.X.; Ye, D.Z.; Liu, G.M.; Yang, X.W. Penigrisacids A-D, four new sesquiterpenes from the deep-sea-derived Penicillium griseofulvum. Mar. Drugs 2019, 17, 507.
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Leshchenko, E.V.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Pislyagin, E.A.; Kim, N.Y.; Berdyshev, D.V.; et al. Piltunines A-F from the marine-derived fungus Penicillium piltunense KMM 4668. Mar. Drugs 2019, 17, 647.
- Shi, Z.Z.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018, 81, 319–325.
- Zhang, J.; Liu, S.S.; Yuan, W.Y.; Wei, J.J.; Zhao, Y.X.; Luo, D.Q. Carotane-type sesquiterpenes from cultures of the insect pathogenic fungus Isaria fumosorosea. J. Asian Nat. Prod. Res. 2017, 21, 234–240.
- Li, Y.L.; Liu, W.; Xu, W.; Zeng, X.; Cheng, Z.B.; Li, Q. Aspterrics A and B, new sesquiterpenes from deep sea-derived fungus Aspergillus terreus YPGA10. Rec. Nat. Prod. 2020, 14, 18–22.
- Liu, X.H.; Hou, X.L.; Song, Y.P.; Wang, B.G.; Ji, N.Y. Cyclonerane sesquiterpenes and an isocoumarin derivative from the marine-alga-endophytic fungus Trichoderma citrinoviride A-WH-20-3. Fitoterapia 2020, 141, 104469.
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572.
- Fang, S.T.; Wang, Y.J.; Ma, X.Y.; Yin, X.L.; Ji, N.Y. Two new sesquiterpenoids from the marine-sediment-derived fungus Trichoderma harzianum P1-4. Nat. Prod. Res. 2019, 33, 3127–3133.
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559.
- Jiang, C.X.; Li, J.; Zhang, J.M.; Jin, X.J.; Yu, B.; Fang, J.G.; Wu, Q.X. Isolation, identification, and activity evaluation of chemical constituents from soil fungus Fusarium avenaceum SF-1502 and endophytic fungus Fusarium proliferatum AF-04. J. Agric. Food Chem. 2019, 67, 1839–1846.
- Zhang, M.; Zhao, J.L.; Liu, J.M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Feng, K.P.; Zhang, D.; Dai, J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017, 19, 651–658.
- Song, Y.P.; Miao, F.P.; Liu, X.H.; Yin, X.L.; Ji, N.Y. Cyclonerane derivatives from the algicolous endophytic fungus Trichoderma asperellum A-YMD-9-2. Mar. Drugs 2019, 17, 252.
- Song, Y.P.; Liu, X.H.; Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Ji, N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry 2018, 152, 45–52.
- Qin, D.; Wang, L.; Han, M.J.; Wang, J.Q.; Song, H.C.; Yen, X.; Duan, X.X.; Dong, J.Y. Effects of an endophytic fungus Umbelopsis dimorpha on the secondary metabolites of host-plant Kadsura angustifolia. Front. Microbiol. 2018, 9, 2845.
- Isaka, M.; Yangchum, A.; Supothina, S.; Boonpratuang, T.; Choeyklin, R.; Kongsaeree, P.; Prabpai, S. Aromadendrane and cyclofarnesane sesquiterpenoids from cultures of the basidiomycete Inonotus sp. BCC 23706. Phytochemistry 2015, 118, 94–101.
- Lei, H.; Lin, X.P.; Han, L.; Ma, J.; Ma, Q.J.; Zhong, J.L.; Liu, Y.H.; Sun, T.M.; Wang, J.H.; Huang, X.S. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69.
- Chen, S.C.; Li, H.H.; Chen, Y.C.; Li, S.N.; Xu, J.L.; Guo, H.; Liu, Z.M.; Zhu, S.; Liu, H.X.; Zhang, W.M. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorg. Chem. 2019, 86, 368–374.
- Yang, M.S.; Cai, X.Y.; He, Y.Y.; Lu, M.Y.; Liu, S.; Wang, W.X.; Li, Z.H.; Ai, H.L.; Feng, T. Seco-sativene and seco-longifolene sesquiterpenoids from cultures of endophytic fungus Bipolaris eleusines. Nat. Prod. Bioprospect. 2017, 7, 147–150.
- He, J.; Yang, M.S.; Wang, W.X.; Li, Z.H.; Elkhateeb, W.A.M.; Wen, T.C.; Ai, H.L.; Feng, T. Anti-phytopathogenic sesquiterpenoid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131.
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159.
- Qiu, Y.; Lan, W.J.; Li, H.J.; Chen, L.P. Linear triquinane sesquiterpenoids: Their isolation, structures, biological activities, and chemical synthesis. Molecules 2018, 23, 2095.
- Tabuchi, A.; Fukushima-Sakuno, E.; Osaki-Oka, K.; Futamura, Y.; Motoyama, T.; Osada, H.; Ishikawa, N.K.; Nagasawa, E.; Tokimoto, K. Productivity and bioactivity of enokipodins A-D of Flammulina rossica and Flammulina velutipes. Biosci. Biotechnol. Biochem. 2020, 84, 876–886.
- Liu, H.X.; Tan, H.B.; Chen, K.; Chen, Y.C.; Li, S.N.; Li, H.H.; Zhang, W.M. Cerrenins A-C, cerapicane and isohirsutane sesquiterpenoids from the endophytic fungus Cerrena sp. Fitoterapia 2018, 129, 173–178.
- Ding, J.H.; Li, Z.H.; Wei, K.; Dong, Z.J.; Ding, Z.H.; Feng, T.; Liu, J.K. Two new sesquiterpenoids from cultures of the basidiomycete Tremella foliacea. J. Asian Nat. Prod. Res. 2016, 18, 46–50.
- Chen, Z.M.; Wang, S.L. Two new compounds from cultures of the basidiomycete Antrodiella albocinnamomea. Nat. Prod. Res. 2015, 29, 1985–1989.
- Huang, L.; Lan, W.J.; Li, H.J. Two new hirsutane-type sesquiterpenoids chondrosterins N and O from the marine fungus Chondrostereum sp. Nat. Prod. Res. 2018, 32, 1578–1582.
- Qi, Q.Y.; Ren, J.W.; Sun, L.W.; He, L.W.; Bao, L.; Yue, W.; Sun, Q.M.; Yao, Y.J.; Yin, W.B.; Liu, H.W. Stucturally diverse sesquiterpenes produced by a Chinese Tibet fungus Stereum hirsutum and their cytotoxic and immunosuppressant activities. Org. Lett. 2015, 17, 3098–3101.
- Liu, H.X.; Tan, H.B.; Chen, Y.C.; Li, S.N.; Li, H.H.; Zhang, W.M. Cytotoxic triquinane-type sesquiterpenoids from the endophytic fungus Cerrena sp. A593. Nat. Prod. Res. 2019, 34, 2430–2436.
- Huang, L.; Lan, W.J.; Deng, R.; Feng, G.K.; Xu, Q.Y.; Hu, Z.Y.; Zhu, X.F.; Li, H.J. Additional new cytotoxic triquinane-type sesquiterpenoids chondrosterins K-M from the marine fungus Chondrostereum sp. Mar. Drugs 2016, 14, 157.
- Chen, Z.M.; Chen, H.P.; Wang, F.; Li, Z.H.; Feng, T.; Liu, J.K. New triquinane and gymnomitrane sesquiterpenes from fermentation of the basidiomycete Antrodiella albocinnamomea. Fitoterapia 2015, 102, 61–66.
- Wang, M.; Du, J.X.; Yang, H.X.; Dai, Q.; Liu, Y.P.; He, J.; Wang, Y.; Li, Z.H.; Feng, T.; Liu, J.K. Sesquiterpenoids from cultures of the basidiomycetes Irpex lacteus. J. Nat. Prod. 2020, 83, 1524–1531.
- Wang, Y.Z.; Wang, Y.; Wu, A.A.; Zhang, L.; Hu, Z.Y.; Huang, H.Y.; Xu, Q.Y.; Deng, X.M. New 12,8-eudesmanolides from Eutypella sp. 1–15. J. Antibiot. 2017, 70, 1029–1032.
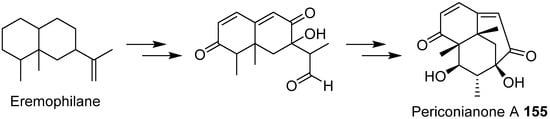
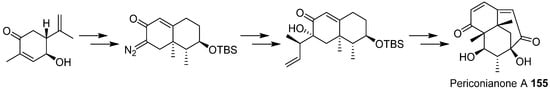
- Zhang, D.W.; Ge, H.L.; Zou, J.H.; Tao, X.Y.; Chen, R.D.; Dai, J.G. Periconianone A, a new 6/6/6 carbocyclic sesquiterpenoid from endophytic fungus Periconia sp. with neural anti-inflammatory activity. Org. Lett. 2014, 16, 1410–1413.
- Liffert, R.; Linden, A.; Gademann, K. Total synthesis of the sesquiterpenoid periconianone A based on a postulated biogenesis. J. Am. Chem. Soc. 2017, 139, 16096–16099.
- Zhao, Z.Z.; Liang, X.B.; Feng, W.S.; Wu, Y.; Zhi, Y.L.; Xue, G.M.; Chen, H.P.; Liu, J.K. Unusual constituents from the medicinal mushroom Ganoderma lingzhi. RSC Adv. 2019, 9, 36931–36939.
- Binh, P.T.; Descoutures, D.; Dang, N.H.; Nguyen, N.P.; Dat, N.T. A new cytotoxic gymnomitrane sesquiterpene from Ganoderma lucidum fruiting bodies. Nat. Prod. Commun. 2015, 10, 1911–1912.
- Wang, J.F.; He, W.J.; Kong, F.D.; Tian, X.P.; Wang, P.; Zhou, X.J.; Liu, Y.H. Ochracenes A-I, humulane-derived sesquiterpenoids from the antarctic fungus Aspergillus ochraceopetaliformis. J. Nat. Prod. 2017, 80, 1725–1733.
