1. Introduction
Multiple Sclerosis (MS) is a serious systemic demyelinating disease that leads to the partial paralysis of the patient who needs the assistance of medicare in order to survive with low quality of life. The need for an effective treatment in the form of medication or vaccine is more urgent than ever before [1,2,3,4,5]. This chronic inflammatory and neurodegenerative disease is initiated by autoreactive T helper (Th) cells and affects approximately 2.5 million people worldwide. Thus, there is an urgent need to develop effective treatments. Advances in the immunotherapy of MS have been recently reported in excellent reviews [6,7,8,9]. The pathogenesis of MS has been extensively studied over the last years, which shows a complex immunological involvement. Myelin epitopes have been identified as a target for autoimmune CD4+ T cells and antibodies, and much focus has been around the modulation of Th1 pro-inflammatory autoreactive CD4+ T cells against myelin epitopes, namely myelin basic protein (MBP), proteolipid protein (PLP), and myelin oligodendrocyte glycoprotein (MOG) [10,11,12,13,14,15,16,17,18].
2. Applied Strategies Utilized against MS
2.1. Linear Epitopes and Derivatives of Selective MBP Epitopes
Linear myelin epitopes MBP
83–99, MBP
82–98, MBP
85–99, MBP
87–99 (
Figure 1 and
Figure 2), MOG
35–55, and PLP
139–151 have been identified as agonist peptides inducing disease in humans and in animal models of MS. These peptides bind to MHC class II alleles, however, peptides binding to MHC class I have also been identified, primarily HLA-A*0301 (HLA-A3) in complex with a PLP
45–53 peptide and the crystal structure known (
Figure 3). Mutant analogs of linear agonist MHC class II peptides have been used to obtain information on the molecular basis of the disease. Data points out that mutant analogs of disease-associated epitopes can inhibit disease through two distinct mechanisms, one via the activation of antigen-specific regulatory T cells, or two, by activation and secretion of appropriate cytokines. The application of MBP
83–99-based altered peptide ligands inhibits MBP-reactive T cell proliferation in vitro [
30]; this is attributed to anti-inflammatory Th2 cytokine secretion by T cells, primarily IL-4 and IL-10. These obtained results point out that cytokine regulation is the major mechanism through which T-cell receptor (TCR)-specific CD4+ T cells regulate encephalitogenic and potentially other bystander Th1 cells. Thus, the modulation of cytokine secretion by auto-reactive T cells through peptide or non-peptide mimetics, even in longstanding autoimmune disease through cytokine therapy, might be beneficial therapeutically. This beneficial response is achieved by switching the function of myelin reactive T cells.
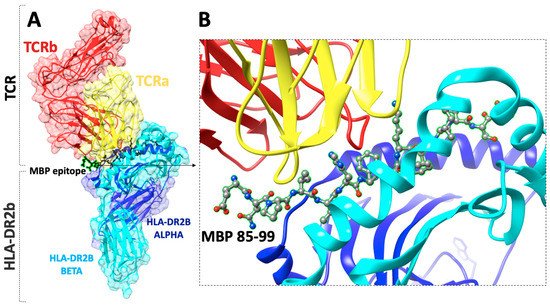
Figure 1. (A) The X-ray structure (pdb: 1YMM) of a human autoimmune TCR bound to a myelin basic protein (MBP85–99) peptide and a MS-associated MHC class II molecule (HLA-DR2b), (B) close view of the docking site of (MBP85–99) peptide.
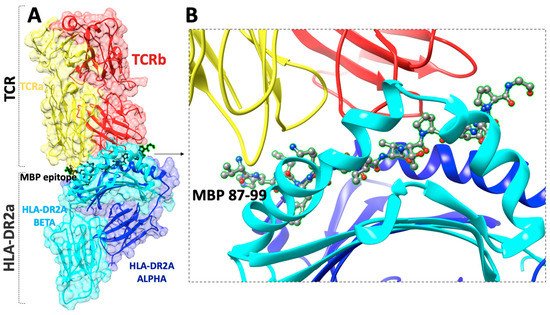
Figure 2. (A) The X-ray structure (pdb: 1ZGL) of a human autoimmune TCR bound to a myelin basic protein self-peptide (MBP87–99) and a multiple sclerosis-associated MHC class II molecule (HLA-DR2a), (B) close view of the docking site of (MBP87–99) peptide.
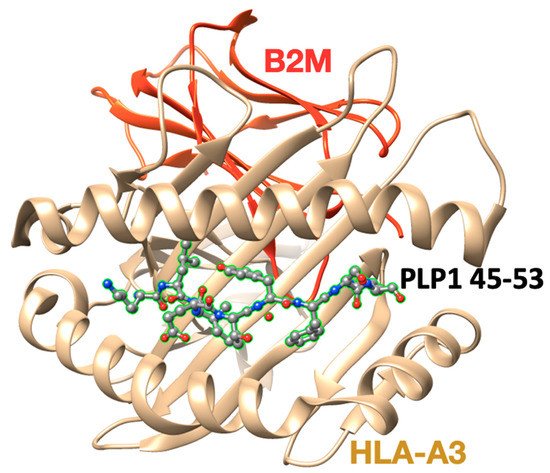
Figure 3. The X-ray crystal structure (pdb: 2XPG) of the human major histocompatibility (MHC) class I molecule HLA-A*0301 (HLA-A3) in complex with a PLP45–53 peptide.
One of the first human clinical studies for patients with secondary progressive MS (MAESTRO-01) used the agonist MBP
82–98 peptide (dirucotide). Intravenous injection of MBP
82–98 peptide delayed disease progression. However, this effort was suspended at phase III stage since it lacked efficacy and did not meet primary endpoints [
40,
41].
Two mutant peptides namely [R
91, A
96]MBP
87–99 and [A
91,A
96]MBP
87–99 derived from the immunodominant agonist identified in MS (MBP
87–99) were synthesized. The chosen mutations occurred at amino acids K
91 and P
96 as they are critical TCR contact sites. Immunization of mice with these altered peptide ligands (ALPs) emulsified in complete Freund’s adjuvant induced both IFNγ and interleukin-4 (IL-4) responses while the native MBP
87–99 peptide induced only IFNγ responses. The linear MBP
72–85 peptide (EKSERSEDENPV) is well known to induce experimental autoimmune encephalitis (EAE), and D
79 mutation with A
79 resulted in an analog that suppressed the induction of EAE [
22]. In addition, the immunodominant peptide from PLP, HSLGKWLGHPDKF, is a naturally processed epitope. A double mutation of the agonist PLP
139–151, in which both of the TCR binding sites are replaced with Leu or Arg ([L
144, R
147]PLP
139–151), is able to antagonize PLP-specific T cell clones in vitro [
42]. The mutated analog inhibited EAE and prevented clinical disease progression when administrated in the early stage of EAE induction [
42]. Antibodies against the minor protein MOG have been noted in inflammation areas of MS. This proves that antibodies do play a role in MS and cooperate with antigen-presenting cells in myelin destruction. Blocking the effects of these MOG antibodies with secondary antibodies or non-peptide mimetics might be an important avenue for future therapy.
2.2. Cyclization of Selective Linear Epitopes and Their Derivatives
Linear peptide are known to be sensitive to proteolytic enzymes, which results in their degradation. Cyclization of linear peptides increases their stability in vitro and in vivo [
43]. In an attempt to develop non-peptide mimetics, cyclic peptides are an important intermediate step towards this. As such, cyclic counterparts of linear peptides have been synthesized in an effort to improve their biological properties and structural stability [
20,
21,
22,
23,
24,
25,
26]. Cyclic MBP
82–98 exerts strong binding to the HLA-DR2 allele but has lower affinity binding to the HLA-DR4 allele. Cyclic analogues of dirucotide proved to be promising leads, and it is proposed that they be evaluated for their ability to alter T cell responses for therapeutic benefit against MS [
40,
41].
Spectroscopic data combined with Molecular Dynamics (MD) calculations showed that the linear MBP
72–85 peptide adopts a pseudo-cyclic conformation. Based on this information, the cyclic analogue QKSQRSQDQNPV-NH
2 was rationally designed. The cyclization of this molecule was achieved by connecting the side-chain amino and carboxyl groups of Lys and Glu at positions 2 and 9. This cyclic analogue exerted similar biological activity to the linear peptide; however, in EAE experiments, the cyclic analogue completely suppressed EAE by co-injection with the agonist peptide in Lewis rats. The similar potencies propose that cyclization does not substantially affect the conformational properties of its linear analogue and provides support to its proposed pseudo-cyclic conformation. In addition, this study proposes that a pseudo cyclic conformation for the MBP
72–85 epitope allows D
81 and K
78 binding to the trimolecular complex MHC-peptide-TCR, and as a consequence, it inhibits EAE [
23].
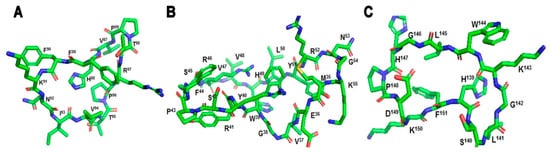
A cyclic analogue, cyclo(87–99)MBP
87–99 (
Figure 4), of the human immunodominant MBP
87–99 epitope, was synthesized based on the same rational as MBP
72–85 epitope. This cyclic analogue in the same manner was shown to mimic the effects of the linear MBP
87–99 epitope peptide, and thus to induce EAE, bind HLA-DR4, and increase CD4 T-cell line proliferation. The mutant cyclic peptides, cyclo(91–99)[A
96]MBP
87–99 and cyclo(87–99)[R
91A
96]MBP
87–99, suppressed, to a varying degree, EAE, and possessed the following immunomodulatory properties: (i) they suppressed the proliferation of a CD4 T-cell line raised from a MS patient; (ii) they scored the best in vitro Th2/Th1 cytokine ratio in peripheral blood mononuclear cell (PBMC) cultures, inducing IL-10 selectively; (iii) they bound to HLA-DR4, first to be reported for cyclic MBP peptides; and (iv) they were found to be more stable to lysosomal enzymes and Cathepsin B, D, and H, compared to their linear counterparts. Such beneficial properties establish these synthetic peptides as putative immunotherapeutics for treating MS and potentially other Th1-mediated autoimmune diseases [
23]. The mutations have been chosen as they identified the major TCR contact sites by X-ray crystallographic studies of human MHC and Molecular Dynamics (MD) studies using murine MHC.
Figure 4. (A) cyclic-MBP87–99 (VHFFKNIVTPRTP), (B) cyclic-MOG35–55 (MEVGWYRSPFSRVVHLYRNGK), (C) cyclic-PLP139–151 (HSLGKWLGHPDKF).
The cyclic-MOG
35–55 peptide, cyclized at the C- and N-terminal amino acids (cyclic-MOG
35–55), altered the 3D conformation of the linear MOG
35–55 peptide (
Figure 4). Following the injection of cyclic-MOG
35–55 during disease induction, EAE, demyelination, and chronic axonopathy in acute and chronic phases of disease were reduced. Molecular docking and spectroscopic data revealed milder interactions between the cycic-MOG
35–55 and mouse or human MHC class II alleles (H2-IA
b and HLA-DR2) [
44]. Likewise, synthesis of cyclic PLP
139–151 peptide and injection in SJL/J mice showed that cylic-PLP
139–151 analog is minimally encephalitogenic when administered to induce EAE (
Figure 4). In particular, cyclic-PLP
139–151 analog showed low disease burden and minimal inflammatory, demyelinating, and axonopathic pathology compared to its linear counterpart. Proliferation assays confirmed the low stimulatory potential of the cyclic-PLP
139–151 compared to linear PLP
139–151 as well as the induction of lower antibody responses. Comparative molecular modeling studies between the two molecules may explain the biological data, as it was shown that different amino acids are involved in the TCR recognition [
42]. It is clear that cyclic modification of linear peptide counterparts may provide novel approaches for future, immunomodulative treatments against MS.
2.3. Conjugation of Selective Epitopes with Mannan
Mannan, a poly-mannose isolated from the cell wall of yeasts, has been shown to exert immunomodulatory effects in the cancer settings in vitro [
45,
46,
47,
48,
49,
50], in vivo (inbred mice, transgenic mice, rats, rabbits, and chickens) [
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62], in rhesus macaques [
63,
64,
65], and in human clinical trials [
66,
67,
68,
69,
70,
71,
72,
73,
74,
75]. Mannan targets antigens to the mannose receptor, antigens endocytosed for MHC class I or II presentation, and modulation of appropriate T cells [
45,
53,
54,
55,
76,
77,
78,
79,
80,
81]. In relation to autoimmune disorders, mannan conjugates (i) represent a new class of immunoregulators that directly and selectively target a population of immune cells that are implicated in the pathogenesis and progression of disease; (ii) provide first line treatment that selectively tolerates or inactivates disease-inducing cells in patients and also prevents progression of disease by stopping diversification of the autoimmune response to additional epitopes; (iii) allows easier formulation of newly discovered molecules within the mannan matrix platform; and (iv) can achieve block-buster status as a global vaccine drug for efficient treatment of MS [
27,
28,
29,
30].
Altered peptide ligands, where one to two amino acid mutations are made to those interacting with the TCR are able to alter an agonist peptide into an antagonist peptide by reduction of hydrogen bond interactions [
82]. Cyclization of peptides allows for their stronger stability and protection against enzymatic and proteolytic degradation [
43]. As such, a cyclic APL, cyclo(87–99)[A
91,A
96]MBP
87–99] reduced Th1 responses, but when conjugated to reduced mannan, an additional significant reduction of Th1 responses and moderate Th2 responses was induced (
Table 1) [
27,
30]. Likewise, APL of linear and cyclic MBP
83–99 analogs, MBP
83–99(A
91,A
96), conjugated to reduced mannan, resulted in diversion of Th1 response to Th2 [
27]. The use of reduced mannan to further divert immune responses to Th2 when conjugated to MBP peptides constitutes a novel strategy for immunotherapy of the disease. The main advantages of mannan conjugates is their stability and non-toxicity. In addition, linear and cyclic peptide analogs based on MBP
83–99 immunodominant epitope conjugated to reduced mannan via (KG)
5 or keyhole limpet hemocyanin (KLH) linkers, were evaluated for their biological/immunological profiles in SJL/J mice. Of all the peptide analogs tested, linear MBP
83–99(F
91) and MBP
83–99(Y
91) conjugated to reduced mannan and cyclic MBP
83–99(F
91) conjugated to reduced mannan yielded the best immunological profile and constitute novel candidates for further immunotherapeutic studies against MS for translation into human clinical trials. Immune responses were diverted from Th1 to Th2 in SJL/J mice and generated antibodies that did not cross-react with native MBP protein. Molecular modeling was used to identify H-bonding and van der Waals interactions between peptides and MHC (I–A
s) [
21]. Furthermore, MBP
87–99(R
91, A
96) conjugated to reduced mannan induced 70% less IFNγ compared with the native MBP
87–99 peptide. However, MBP
87–99(A
91,A
96) conjugated to reduced mannan did not induce IFNγ-secreting T cells, elicited very high levels of IL-4, and antibodies generated did not cross-react with the native MBP
87–99 peptide. It is clear that this double-mutant peptide analog conjugated to reduced mannan is able to divert immune responses from Th1 to Th2 and is a promising mutant peptide analogue for use in studies exploring potential treatments for MS [
21].