Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Glycol chitosan (GC), a water-soluble chitosan derivative with hydrophilic ethylene glycol branches, has both hydrophobic segments for the encapsulation of various drugs and reactive functional groups for facile chemical modifications.
- polymeric nanoparticles
- fluorescence imaging
- antibacterial
- anticancer
- supramolecular self-assembly
1. Introduction
Both cancer and microbial infections are major threats to human health and have become the leading causes of death in the world for decades [1,2]. To date, a variety of systemically delivered drug carriers such as inorganic nanovehicles, liposomes, and polymeric nanoparticles (NPs) have been developed to ferry different types of drugs including small molecules, nucleic acids, and peptides/proteins, with the aim to enhance their therapeutic efficacies against tumors and microbial infections with better biosafety [3]. Among them, polysaccharides have been widely demonstrated as a class of excellent drug carriers because of their good biocompatibility/biodegradability and low cost [4]. Chitosan is a linear polysaccharide derived by the deacetylation of the N-acetyl glucosamine units of chitin, a natural polymer from crustacean shells, through hydrolysis at high temperatures under alkaline conditions. Chitosan has been widely applied in the biomedical field, for it is biocompatible, biodegradable, and abundant in nature. However, chitosan is typically insoluble in water above pH 6 and requires the addition of acid to ensure the protonation of the primary amines, which greatly limits its further modifications for desired purposes. Glycol chitosan (GC) is a chitosan derivative conjugated with hydrophilic ethylene glycol branches that render the polymer soluble in water at a neutral/acidic pH. The molecular weight of GC ranges from 20 to 250 kDa with the degree of deacetylation from 60 to 82.7% [5]. The reactive functional groups of GC such as amine and hydroxyl groups provide flexibility for various chemical modifications to form a number of derivatives. These GC derivatives form self-assembled nanostructures or hydrogels, serving as excellent drug delivery systems for diagnostic agents and therapeutic drugs to combat cancers and pathogenic microorganisms (Figure 1).
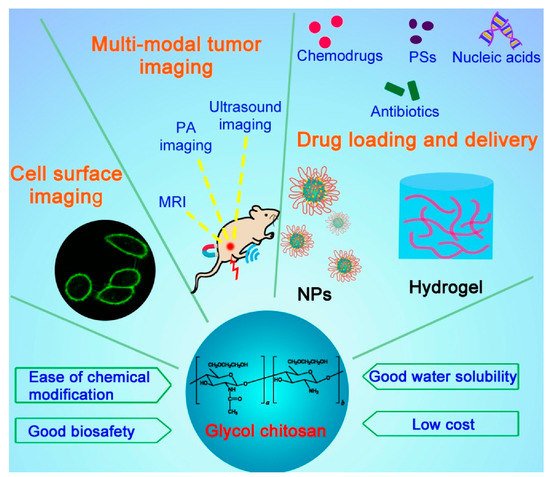
Figure 1. Schematic illustrating the structure, properties, and applications of glycol chitosan.
2. GC Derivatives for Imaging Applications
2.1. Cell Surface Imaging
The plasma membranes of cells are involved in many biological events such as signal transduction, endocytosis, exocytosis, cell migration, cell adhesion, cell proliferation, and apoptosis [6]. Cell surface labeling is a powerful tool to study these plasma membrane-related cellular behaviors. Typically, plasma membrane dyes are small molecules, which can only label the cell surface for a short time period due to their fast cellular internalization characteristic [6]. Therefore, it is necessary to develop a fluorescent dye for stable plasma membrane imaging. Recently, our group has developed a GC-based fluorescent probe by linking GC with cholesterol-polyethylene glycol (PEG-Chol) and fluorescein isothiocyanate (FITC), and successfully applied this probe (termed Chito-Chol-FITC, GC-PEG cholesterol-FITC, or GC-PEG Chol-FITC) to cell surface labeling [6,7,8]. The Chito-Chol-FITC could bind to the plasma membranes of mammalian cells through the insertion of the hydrophobic cholesterol units into the lipid bilayers (Figure 2A). The imaging results revealed that Chito-Chol-FITC rapidly stained the cell membrane within 5 min and resisted cellular internalization for up to 6 h [6]. What is more, Chito-Chol-FITC realized the universal imaging of the plasma membranes of mammalian cells (by hydrophobic interaction) and the cell walls of fungal and bacterial cells (by electrostatic interaction) (Figure 2B) [7]. Notably, Chito-Chol-FITC did not detach from the cell surface after permeabilization treatment during immunofluorescence staining and was compatible with the immunofluorescence staining for the simultaneous labeling of the plasma membrane and cytoskeletons (Figure 2C), ensuring the clear observation of binucleated cells and metaphase cells [8]. The anti-permeabilization property of Chito-Chol-FITC was attributed to its large molecular weight and the amine crosslinking between Chito-Chol-FITC and the membrane proteins/lipids induced by the addition of paraformaldehyde in the fixation step. The imaging performance of Chito-Chol-FITC far surpassed that of the commercial plasma membrane dyes like FM families and DiD, which were internalized by the cells in 10–15 min. In addition to the facile synthesis and low cost of Chito-Chol-FITC, this probe is promising to serve as a novel and valuable platform for studying cell surface-related biological events and advancing cell surface engineering. In another work by the same group, long-time plasma membrane imaging was realized by the supramolecular recognition between GC-10% PEG2000 cholesterol-10% biotin (GC-Chol-Biotin) and FITC-conjugated avidin (avidin-FITC) (Figure 2D) [9]. This two-step strategy presented a stable plasma membrane imaging for up to 8 h without substantial internalization or detachment of the dyes, and outperformed the current commercial plasma membrane imaging agents such as CellMask and DiD. Furthermore, the authors used this imaging method to dynamically monitor different plasma membrane behaviors including plasma membrane vesiculation, membrane blebbing, and cell shrinkage. Besides being used to image the whole plasma membranes, GC was also designed to visualize specific membrane structures, e.g., lipid raft domains, by grafting GC with FITC [10]. In summary, GC polymers can serve as a promising platform for constructing various cell surface probes with desirable properties, benefiting from their good water solubility, ease of chemical modification, and low cytotoxicity.
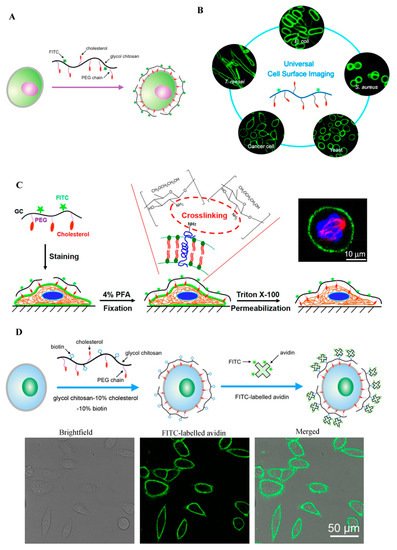
Figure 2. GC-based cell surface imaging. (A) Schematic displaying the plasma membrane labeling of Chito-Chol-FITC. Reproduced with permission from Ref. [6]. Copyright 2015 Royal Society of Chemistry. (B) Schematic illustration of the universal cell surface imaging of animal cells, bacteria, and fungi using Chito-Chol-FITC. Reproduced with permission from Ref. [7]. Copyright 2016 American Chemical Society. (C) Mechanistic diagram of the anti-permeabilization property of Chito-Chol-FITC during immunofluorescence staining. Reproduced with permission from Ref. [8]. Copyright 2017 American Chemical Society. (D) Schematic illustration of long-time cell membrane labeling using a two-step modification method through the recognition of FITC-labeled avidin with biotin. Reproduced with permission from Ref. [9]. Copyright 2016 American Chemical Society.
2.2. GC Derivatives for In Vivo Cancer Diagnosis
Detecting cancers at the early stages before their metastasis occurs through the lymph systems and blood vessels is very important for cancer treatments, but still remains challenging. To date, different noninvasive imaging modalities like X-ray, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), positron emission tomography (PET), optical fluorescence imaging, and single photon emission computed tomography (SPECT) have been employed for the detection of cancers in the early stages [11]. These imaging strategies are also critical for the discovery and development of novel drugs by monitoring drug responses and distributions in real time and measuring biological changes in living systems. Recently, GC and its derivatives have been commonly used to modify or load various imaging agents, e.g., iron oxide NPs [12,13] and gadolinium [14], for improving their cancer diagnostic outcomes. In 2011, Yuk et al. prepared a GC/heparin-immobilized iron oxide NPs (composite NPs) to achieve MRI [12]. Since both iron oxide NPs and GC NPs are cationic, gold was deposited on the surface of the cationic iron oxide NPs to introduce negative charges on the surface, ensuring the electrostatic interaction between the iron oxide NPs and GC NPs in the aqueous media. Gold-deposited iron oxide NPs were immobilized into the GC/heparin network to form the composite NPs. The composite NPs were stabilized with heparin via the electronic interaction between cationic GC and anionic heparin. The composite NPs showed improved T2* negative images as compared with Resovist, and realized highly selective tumor imaging.
Meanwhile, GC has been used to coat gold NPs (GC-AuNPs) as an imaging contrast agent for the US-guided photoacoustic (USPA) imaging of sentinel lymph node (SLN) metastases [15]. The USPA immunofunctional imaging successfully discriminated metastatic lymph nodes from non-metastatic ones [15], which can help physicians to detect micrometastases by SLN biopsy. GC-AuNPs were conjugated with 4-azidobenzoic acid (AzBA), yielding AzGC-AuNPs [16]. AzBA groups produced nitrogen gas (N2) through photolysis to form echogenic N2 microbubbles for the enhanced contrast in US imaging. Owing to their small size (less than 100 nm), AzGC-AuNPs could penetrate the endothelial barrier for diagnosis of diseases, which outperformed conventional microbubbles. Together with the generated negative zeta potentials after gas generation, this small size of AzGC-AuNPs brought about excellent blood residency and clearance. Therefore, AzGC-AuNPs have great potential to serve as a US contrast agent for US imaging and other contrast-enhanced imaging methods such as MRI and optical coherence tomography. One limitation of AzGC-AuNPs is its optical absorption at low wavelengths (i.e., 520–530 nm), which can be solved by using gold nanorods or other particles with absorption in the near-infrared (NIR) region.
Multimodal imaging can provide more credible and accurate imaging of focal areas by overcoming the limitations of single-modal imaging. MRI is one of the most powerful diagnostic approaches for three-dimensional human body imaging with high spatial resolution and deep tissue penetration. However, MRI shows low sensitivity to contrast agents, requiring a long time and a high dose of the agent for effective imaging. By contrast, optical imaging with NIR fluorescence (NIRF) allows a rapid screening of diseases with a low dose, but has poor tissue penetration. Consequently, the dual-modal imaging via NIRF imaging and MRI is believed to overcome the intrinsic disadvantages of single-modal NIRF imaging and single-modal MRI. For example, Nam and co-workers developed an NIRF and MRI dual-modality imaging agent using GC NPs, an effective positive MRI contrast agent (gadolinium), and NIRF dye (cyanine 5.5, Cy5.5) [14]. 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) for gadolinium chelating was conjugated to GC-5β-cholanic acid (GC-CA) to generate Cy5.5-GC-CA NPs. Cy5.5-GC-CA NPs contained up to 6.28 wt% gadolinium and successfully visualized tumor in the T1-weighted MR image as well as the NIRF image. In addition, these NPs displayed long-term and stable blood circulation and high tumor selectivity due to the enhanced permeability and retention (EPR) effect of tumor tissues. This optical/MR dual imaging probe offered an excellent spatial resolution of tumor tissues with high sensitivity, presenting a promising way for cancer imaging. Nevertheless, gadolinium-based contrast agents can trigger the development of nephrogenic systemic fibrosis, a fibrosing disorder found in patients with renal impairment. Thus, superparamagnetic iron oxide NPs (SPIOs) were utilized as an alternative to gadolinium [13]. The NIRF and MRI dual-modality imaging system using GC, Cy5.5, and SPIOs was effective in displaying tumor regions in T2-weighted images with high tumor specificity. These results demonstrated the feasibility of using GC NP-based optical/MR dual imaging for the early cancer detection. Besides optical/MR dual-modality imaging, GC NPs might also be used for various multimodal diagnostic imaging such as optical/CT, optical/US, and optical/PET, and even for trimodal imaging to increase the opportunities for the successful early diagnosis of tumors.
3. GC Derivatives for Drug Delivery
3.1. GC Derivatives for the Delivery of Antimicrobial Agents
GC is water-soluble, biocompatible, and biodegradable, serving as a good carrier to deliver antimicrobial agents in active forms for the applications in wound dressing, tissue adhesive, and hemostasis [17]. GC-derived NPs [18,19,20], microspheres [21], and hydrogels [22,23] have been utilized to carry various antibiotics like colistin [22], chlorhexidine acetate [21], and ciprofloxacin [23], silver ions [20], and photothermal therapy (PTT) agents [18,19,23]. Recently, several studies have reported the applications of GC derivatives for antimicrobial PTT using NIR light [18,19,23]. For instance, a pH-sensitive NP system consisting of polyaniline-conjugated GC (PANI-GCS) was developed [18]. This system specifically gathered pathogenic bacteria in the acidic abscesses and destroyed them photothermally by NIR light. Hydrophobic PANI, a PTT agent, was covalently conjugated with GC via its highly reactive amine groups to generate an amphiphilic polymer (PANI-GCS) that self-assembled into NPs in an aqueous solution. Under normal physiological conditions, the PANI-GCS NPs had neutral surface charges and poorly bound to the neighboring host cells, but became positively charged at the sites of acidity-associated focal infections and strongly interacted with the negatively-charged bacterial cell walls via electrostatic interaction. Thus, the bacteria were aggregated in situ and eradicated under the irradiation of NIR light through the PANI-induced PTT. Meanwhile, this approach reduced the damage to tissues and accelerated wound healing by lowering the ambient tissue temperature. Similarly, another PTT compound carboxyl graphene was grafted with GC to realize an enhanced photothermal treatment of focal infections [19]. In a recent work, our group has fabricated a thermo-sensitive hydrogel by mixing ciprofloxacin (Cip, a potent antibiotic)-loaded polydopamine (PDA) NPs and GC, and the resultant hydrogel (termed Gel-Cip) was injectable and could be used for on-demand antibiotic release to combat bacterial infection (Figure 3) [23]. Bacteria were trapped on the surface of Gel-Cip due to the positive charges of GC and the adsorbability of PDA NPs, and killed synergistically by the Cip released from Gel-Cip and local hyperthermia generated by PDA NPs upon NIR light irradiation. Furthermore, Gel-Cip exhibited an outstanding wound healing ability in the Staphylococcus aureus-infected mouse skin defect model. This hydrogel-based light-activatable system allows for precise antibiotic release, effective bacterium inactivation, and persistent inhibition of bacteria-induced infections.
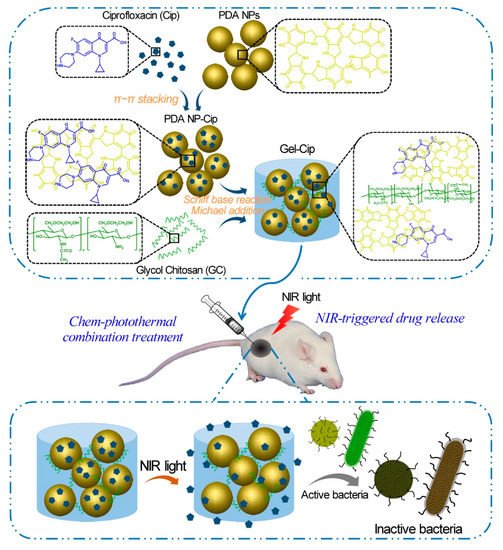
Figure 3. Schematic of the synthetic strategy and bacterial inactivation of Gel-Cip. Reproduced with permission from Ref. [23]. Copyright 2018 Elsevier Ltd.
3.2. GC Derivatives for Anticancer Drug Delivery
Hydrophilic GC is usually conjugated with hydrophobic molecules through various chemical reactions to form amphiphilic GC derivatives, also termed hydrophobically modified GC. Generally, these derivatives can self-assemble in aqueous solutions into NPs that contain inner hydrophobic cores and outer hydrophilic shells. The resultant GC-based NPs can easily incorporate various types of drugs (e.g., hydrophobic anticancer drugs, peptides, and nucleic acids) by hydrophobic/electrostatic interactions or chemical conjugation, and have been intensively employed for cancer theranostics over the past two decades. Recently, hydrogels formed by GC have also attracted increasing attention in drug delivery applications.
This entry is adapted from the peer-reviewed paper 10.3390/molecules24234371
This entry is offline, you can click here to edit this entry!
