Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Diverse medicinal plants such as those from the genus Artemisia have been employed globally for centuries by individuals belonging to different cultures. Universally, Artemisia species have been used to remedy various maladies that range from simple fevers to malaria.
- Artemisia
- extraction
- isolation
- bioactive compounds
1. Introduction
The beauty of natural products chemistry can be witnessed in the sheer diversity of its sources. Plants, animals, and microorganisms contain a vast quantity of bioactive compounds. Plants specifically present remedies for the treatment of various anthropogenic ailments in several continents around the globe such as in Asia, Africa, and South America [1]. Traditional medicine involving plants is used by 80% of the global population and serves as the primary healthcare system [2]. The role compounds derived from natural sources play in the drug discovery process can never be overemphasized. Artemisia has indiscriminately served as a treatment for maladies such as viruses, malaria, bacteria, hepatitis, fungi, cancer, and inflammation [3]. Thus, the biodiversity of nature is something that is to be cherished and upheld. Among the many appealing aspects of biodiversity, the biodiversity of nature signifies numerous untapped opportunities to discover novel plant secondary metabolites. These secondary metabolites originating from flora serve the plant by protecting it against pathogens and herbivores. The primary classes of secondary metabolites for Artemisia species are phenolic compounds, terpenoids, and alkaloids, coumarins, acetylenes, sterols, and caffeoylquinic acids [1][2].
With the distinctive therapeutic and medicinal properties that Artemisia L. possesses, it would be quite remiss to not discuss its taxonomic classifications to arrive at a more holistic perspective of its scope and potential. The genus, Artemisia L., is considered to be one of the largest enumerable and dispersed genera in existence [2]. It is a member of the Asteraceae family (Compositae), a sizable taxonomic classification, which encompasses approximately 1000 genera and 20,000 species [3]. Artemisia is a member of the Anthemideae tribe, which is inclusive of over 500 species; these species are predominantly located in geographic regions such as North America, Europe, and Asia [3]. The species of Artemisia are characterized by their annual, biannual, and perennial herbs or compact shrubs.
A superb advantage of the Artemisia genus is its ability to thrive and persist in nearly all habitat types. To better comprehend the primacy of Artemisia, it is worthwhile to recognize its global presence. Artemisia is indigenous to Europe, North America (United States and Canada), South America (Brazil) Southeast Asia, South Africa, and the Pacific Islands [4]. The preferred climate for this genus is arid and semi-arid. With the exception of Antarctica, Artemisia is incontrovertibly well dispersed on the global arena [5]. Its far-reaching distribution allows for its unique morphology and characteristics [5]. Artemisia effortlessly adapts to a wide range of temperatures but thrives in moist soils [4]. The capability of this species to be able to adapt and survive in both warm and cool temperature environments provides a highly valuable protective mechanism against extinction. Field pictures of eight Artemisia species are present in the Figure 1.

Figure 1. Field pictures of eight Artemisia species [6]: (a) Artemisia absinthium, (b) Artemisia annua, (c) Artemisia biennis, (d) Artemisia campestris, (e) Artemisia douglasiana, (f) Artemisia dracunculus, (g) Artemisia tridentata, and (h) Artemisia vulgaris.
Traditional and Current Uses of Artemisia Species
Artemisia absinthium L. is typically referred to as wormwood and is a perennial plant that is dispersed and distinguishable across parts of Siberia and Europe [3]. It produces vibrant yellow flowers and possesses a wide array of uses. For instance, its antiparasitic properties allow for the treatment of anorexia and indigestion [3]. Moreover, the aerial parts of this shrub can serve as a component in various gastric herbal concoctions, intoxicating beverages, and dietary supplements [3]. In the ethnopharmacological sphere, Artemisia absinthium L. has functioned to restore diminished mental performance, to alleviate liver inflammation, and to enhance memory [2]. The antioxidant activity of the aerial components of A. absinthium has been evaluated by measuring the free-radical scavenging activity of A. absinthium extracts to remove reactive hydroxyl radicals and stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical “during the Fenton reaction trapped by 5,5-dimethyl-1-pyrroline-N-oxide”, along with the use of electron spin spectroscopy [2]. Prior research has conveyed that crude ethanol and aqueous extracts of the aerial parts of A. absinthium display anthelmintic activity when juxtaposed to the effectiveness of albendazole “against the gastrointestinal nematodes of sheep” [2].
Artemisia annua L. is commonly known as “huanghuahao” (yellow flower Artemisia) and it distributes from north to south to almost all parts of China [7]. It is also native to Tanzania, Kenya, India, Romania, Inner Mongolia and Hebei provinces and Vietnam [8]. In Chinese culture, A. annua has been utilized to address fever and chills since the second century B.C. [3][7]. This plant has been introduced to disparate parts of the world. For example, in Africa, A. annua is cultivated and employed in tea form to treat malaria [3]. A clandestine research project known as Project 523 was postulated in a meeting on 23 May 1967, amid the Vietnam War [7]. Evidently, the death toll experienced by North Vietnamese soldiers during battle was eclipsed by the number of soldiers that met their premature demise by malaria. Thus, the search began to discover antimalarial compounds for both the Chinese citizens and the North Vietnamese soldiers. In 1972, a year that marked a turning point for the health of a nation, artemisinin was extracted and recognized for its highly efficacious anti-malarial activity from A. annua [7]. Initially, the potency of this anti-malarial compound, artemisinin, was trivialized by the Western world [7]. Nonetheless, artemisinin repeatedly demonstrated its effectiveness against schistosomiasis and malaria [7]. In addition to A. annua’s antimalarial efficacy, it has been proposed that A. annua can also effectively combat human immunodeficiency virus (HIV) [9]. Therefore, more recent research investigations are involved in determining its antiviral activity against HIV [9]. This is especially pertinent because HIV is a prolific global disease.
Artemisia biennis is typically known as biennial wormwood and is an annual or biennial species naturally present in western North America [10]. The spreading of A. biennis throughout North America occurred as a result of anthropogenic activities such as transportation [10]. The proliferation of A. biennis in agricultural lands can be ascribed to a forbearance of some classes of herbicides, shift in annual growth patterns, crop diversification, and a rise in reduced tillage systems usage [10][11]. A. biennis thrives in highly disturbed habitats and can effortlessly outperform species native to a certain habitat. In this fashion, biennis is considered an invasive species. Although this herb can be quite a quandary in certain regions, it does possess some beneficial uses. This species commonly functions as an antiseptic in folk remedies and also used for cooking purposes [9]. Furthermore, A. biennis has been employed by the indigenous people of North America as ointments and medicinal cleansers for the treatment of wounds, sores, and chest infections [3]. In Iran, the essential oil of this herb is primarily composed of camphor [12]. However, in Western Canada, the most copious volatile compound in the aerial portions of A. biennis is €-β-farnesene [8][12]. Mojarrab et al. (2016) carried out an investigation that sought to determine the efficacy of fractions derived from A. biennis hydroethanolic extract to grant “cytoprotection against oxidative stress and apoptosis induced by doxorubicin (DOX) in rat pheochromocytoma cell line (PC12)” [8][12]. This investigation corroborated that oxidative stress injury as well as “apoptosis induced by DOX in the PC12 cells” was attenuated by the hydroethanolic extract of A. biennis [8][12].
Artemisia campestris L. is frequently referred to as tgouft and is a faintly fragrant perennial herb widely distributed in the south of Tunisia, North Africa, North America, and Eurasia [3][10][13]. The leaves and flowers of A. campestris were conventionally implemented for the treatment of high cholesterol levels, obesity, hypoglycemia, and venin [13]. Innumerable studies attest to A. campestris’s bioactivity. The biological activities of this plant include allelopathic, anthelmintic, antimicrobial, antidiabetic, hepatoprotective, insecticidal, nephroprotective, antivenomous, antiulcer, antioxidant, and antitumor [13]. The study conducted by Ivanescu et al. (2018) on Artemisia campestris from Romania divulged novel bioactive compounds such as acacetin, casticin, eupatorin, stigmasterol, α-sitosterol, gentisic acid, and campesterol through various biological assays and LC-MS [10][13].
Artemisia douglasiana L. is commonly referred to as California mugwort and is a perennial herb native to the Western United States, particularly in northern California, Washington, and Oregon [9]. In Cuyo, a region in which A. douglasiana is nonnative, it is cultivated and used in traditional medicine [3]. The natives of Cuyo refer to A. douglasiana as “matico”; it is employed for the treatment of gastrointestinal disorders and peptic ulcers [3]. Furthermore, Artemisia douglasiana serves as a tonic to remedy nervous disorders and to stimulate menstruation [9]. The essential oil of A. douglasiana has been utilized for aromatherapy, relieving tense muscles, mollifying mental anguish, and inhalation to enhance mental clarity [9]. According to Setzer et al. (2004), the leaf oil from A. douglasiana analyzed by GC-MS revealed the prime components of the oil to be 29% camphor, 26% Artemisia ketone, 13% Artemisia alcohol, 10% alpha-thujone, 8% 1,8-cineole, and 15% hexanal [12][14].
Artemisia dracunculus L. is also known as “tarragon” and is considered a perennial herb widely distributed in western North America, eastern and central Europe, and a majority of temperate Asia [15]. A. dracunculus is also cultivated in India, Russia, Iran, and Ukraine [16]. This herb possesses an extensive background in culinary practices. In America, tarragon is used in seafood, chicken, eggs, tartar sauce, and vinegar [15]. In France, tarragon is used to flavor French Dijon mustard, sour cream, eggs, and mayonnaise [15]. Due to its diverse and expansive health promoting properties, it typically functions as herbal medicine [3]. For instance, extracts of A. dracunculus have been made use of by Himalayan natives to pacify toothache, relieve fever, and to remedy gastrointestinal issues [9]. Tarragon possesses phenomenal antibiotic activity; in Tibetan medicine, this plant is used to treat chronic bronchitis, pulmonary tuberculosis, and pneumonia [16]. Native American Chippewa tribe utilized the roots of tarragon to mitigate superfluous flow during the menstruation period and to assist in strenuous labor [15]. The dominant essential oil components of A. dracunculus consist of coumarins, flavonoids, and phenolic acids [15].
Artemisia tridentata Nutt. is informally known as “basin big sagebrush” and is a perennial shrub that possesses a vast range of distribution [9]. This shrub is native to the semiarid Southwestern United States [17]. Artemisia tridentata encompasses the following subspecies: tridentata, vaseyana, wyomingensis, rupicola, xericansis, scopulorum, and thermopola. A. tridentata serves as a consequential habitat for a great variety of fauna [9][14]. Big sagebrush boasts an assortment of uses. Native Americans utilize this shrub to remedy flu and colds [17]. Historically, A. tridentata has been employed for the alleviation of fevers, poisoning, and gastrointestinal issues. Although basin big sagebrush is frequently lauded for its appreciable chemotherapeutic and bactericidal activities, it is an efficacious aeroallergen and liable to cause dermatitis [17]. The most salient compounds present in A. tridentata include sesquiterpenes, flavonoids, monoterpenes, and coumarins [17].
2. Extraction, Isolation, Characterization
The aim of this review is to address the process involved in order to obtain pure compounds from crude plant extracts, which includes the combination of extraction/sample preparation tools and analytical techniques, for isolating and characterizing bioactive compounds from plants, as potential lead compounds in the drug discovery process. A flowchart of stages involved in extraction, isolation and characterization of bioactive compounds from Artemisia is present in Figure 2.
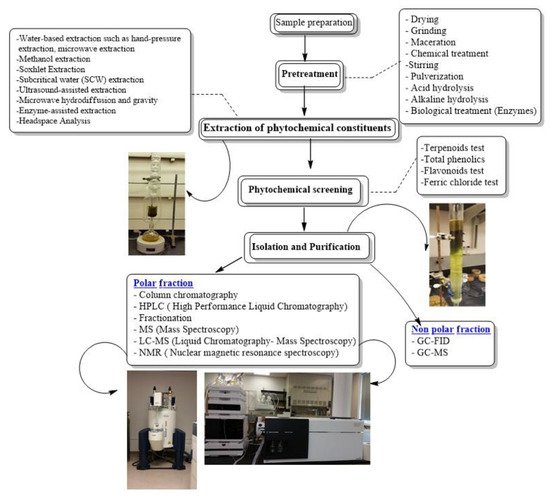
Figure 2. Flowchart of stages involved in extraction, isolation and characterization of bioactive compounds from Artemisia.
2.1. Extraction Techniques
Fauna and flora possess an extensive array of bioactive compounds that can be applied to the enhancement of human wellbeing. However, the extraction of these bioactive compounds by various research groups can be carried out in a more sustainable fashion that can preserve the abundance and richness of the species used in research. Conventional extraction methodologies are entrenched in the employment of vast quantities of organic solvents only to result in less than satisfying extraction yields [18]. Thus, it is imperative and worthwhile for green extraction techniques to be explored. Such techniques prioritize the avoidance of toxic solvents and the increase of extraction yields with minimal energy expenditure. Currently, supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE) are ubiquitous high-pressure techniques for the elution of bioactive compounds from natural sources [18]. These techniques possess considerably high extraction efficiencies [18]. Some of the extraction technique used for the extraction of bioactive compound of Artemisia species has been mentioned in Table 1.
Supercritical fluid extraction (SFE) makes use of a specific solvent under temperatures and pressures beyond its critical point. Under such conditions, the fluid undergoes “physicochemical changes” that impart alterations to its solvent properties [18]. Consequently, no phase separation is observed, and a homogenous supercritical fluid is obtained. This enables supercritical fluids to achieve viscosities akin to gaseous substances while simultaneously retaining densities homologous to liquid substances. These characteristics, in conjunction with others, are what distinguish supercritical fluids from more orthodox solvents found under ambient parameters.
The considerably low concentrations of bioactive compounds in Artemisia species presents a profound challenge that can sometimes derail the progress of their analysis. The extraction of bioactive compounds free of interferents using conventional methods is nearly unattainable. Hydrophilic deep eutectic solvents (DESs) have captured the attention of many and are considered green alternatives for the efficient extraction of bioactive compounds [19]. In an investigation carried out by Jun Cao et al. (2017), the merits of DESs are illuminated and compelling incentives for the use of DESs are discussed. A considerable amount of DESs is typically composed of two or more non-noxious, non-flammable, biodegradable, and inexpensive components that interact with one another via hydrogen bonding [19]. Moreover, deep eutectic solvents possess a wide range of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) which make them applicable in a wide array of fields due to their minimal toxicity, simple preparation, biodegradability, and unique properties [19].
The fundamental intent of this study was to evaluate whether DESs could improve the concentration of artemisinin extracted from Artemisia annua, thereby improving the extraction efficiency. To achieve this end, a series of “screening and tailoring” was carried out to determine which DES would produce the greatest extraction yield30. Subsequently, a hydrophobic DES known as N81Cl-NBA was chosen as the solvent for extraction due to its high extraction yield. N81Cl-NBA was prepared from methyl trioctyl ammonium chloride and 1-butanol using a molar ratio of 1:4 [19]. N81Cl-NBA-based ultrasound-assisted extraction was employed, and the components influencing extraction yield were statistically optimized [19]. Jun Cao et al. (2017) concluded that N81Cl-NBA-based ultrasound-assisted extraction along with “macroporous resin separation” demonstrated a higher extraction efficiency and an artemisinin recovery yield of 85.65% was achieved [19]. Compared to 60–80% yield of artemisinin using conventional organic solvents such as petroleum ether, this is a pleasing improvement [19]. This investigation was able to demonstrate that DESs are “designer solvents” that can be utilized as green extraction solvents for the extraction of bioactive compounds from plant material [19].
Table 1. Bioactive compound extraction techniques for Artemisia species.
| Species Name | Origin | Method | Plant Part | Solvent System | Bioactive Constituent | Percent Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Artemisia annua | Astore, Northern Areas Pakistan | Sonication | Flowers | 5 mL HPLC grade toluene | Artemisinin | 0.42 ± 0.03% | [20] |
| Artemisia annua | Astore, Northern Areas Pakistan | Sonication | Leaves | 5 mL HPLC grade toluene | Artemisinin | 0.44 ± 0.03% | [20] |
| Artemisia annua | Astore, Northern Area Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [20] |
| Artemisia dracunculus var dracunculus | Abbass Pur, Azad Kashmir Pakistan |
Sonication | Leaves | 5 mL HPLC grade toluene | Artemisinin | 0.27 ± 0% | [20] |
| Artemisia dracunculus var dracunculus |
Abbass Pur, Azad Kashmir Pakistan |
Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.12 ± 0.01% | [20] |
| Artemisia parviflora | Rawalakot, Azad Kashmir Pakistan |
Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [20] |
| Artemisia moorcroftiana | Kalam, Swat Pakistan |
Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [20] |
| Artemisia sieversiana | Soost, Northern Areas Pakistan |
Sonication | Roots | 5 mL HPLC grade toluene | Artemisinin | 0.04 ± 0% | [20] |
| Artemisia sieversiana | Soost, Northern Areas Pakistan |
Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [20] |
| Artemisia moorcroftiana | Kalam, Swat Pakistan |
Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [20] |
| Artemisia vestita | Galyat, Pakistan | Sonication | Roots | 5 mL HPLC grade toluene | Artemisinin | 0.04 ± 0% | [20] |
| Artemisia vulgaris | Kalam, Swat Pakistan |
Sonication | Flowers/leaves | 5 mL HPLC grade toluene | Artemisinin | 0.05–0.15% | [20] |
| Artemisia vulgaris | _ | silica gel column chromatography using gradient elution | Leaves | Ethyl acetate and dichloromethane | Yomogin and 1,2,3,4-diepoxy-11(13) eudesmen-12,8-olide | _ | [21] |
| Artemisia douglassiana | _ | Liquid-liquid extraction | Aerial parts | Ethyl acetate-hexane [1:9] | Dehydroleucodine | _ | [21] |
| Artemisia douglassiana | _ | Liquid-liquid extraction | Aerial parts | Silica gel with hexane-ethyl acetate mixtures | Dehydroparishin-B | _ | [21] |
| Artemisia diffusa | _ | Maceration | _ | n-hexane/ethyl acetate/methanol [1:1:1] |
Tehranolide | _ | [21] |
| Artemisia princeps |
_ | Liquid-liquid extraction | Aerial parts | Dichloromethane fraction column chromatography over silica gel using gradient elution of methanol and dichloromethane | Yomogin | _ | [21] |
| Artemisia ludoviciana | _ | Column chromatography | Aerial parts | Organic phase chromatographed repeatedly on normal-phase silica gel with ethyl acetate and hexane | Guaianolide ludartin | _ | [21] |
| Artemisia caerulescens ssp. cretacea |
_ | Concentrated extract is extracted with boiling water. Aqueous solutions are extracted with chloroform | Flowers | Aluminum oxide column with 5% methanol in chloroform | Santonin | _ | [21] |
2.2. Isolation Techniques: TL
Typically, plant extracts are composed of a mixture of bioactive constituents that need to be isolated for the characterization process [22]. Thin layer chromatography (TLC) is a commonplace technique for the separation of mixtures [22]. TLC analysis is a straight-forward, efficient, and inexpensive method that provides the researcher with immediate results on the quantity of the components present in the mixture [23]. The performance of a TLC analysis can also be employed to corroborate the identity of a compound in a mixture by comparing the retention factor (Rf) of the compound in the mixture with a known compound [23]. Subsequently, the components of the mixture on the TLC plate can be visualized with the use of a UV lamp [22].
In a study conducted by Mohammad Bagher Pasha Zanousi (2012), Agrobacterium rhizogenes promoted the formation of hairy roots in an “Iranian clone of Artemisia annua” to evaluate the production of artemisinin in the newly formed hairy roots by TLC analysis [23]. To begin the TLC analysis, the artemisinin standard was dissolved in HPLC grade acetonitrile to produce a 1000 ppm concentration of artemisinin [23]. The anhydrous extracts were placed in 1 mL of acetonitrile. Additionally, a micropipette was used to spot the silica gel TLC plate. The TLC plate was subsequently placed in a glass TLC chamber containing a solvent system of acetone/hexane with a 3:10 molar ratio [23]. The plate was developed to a height of 3 cm and left to air dry at room temperature [23]. The dry TLC plate was visualized in an iodine tank [23]. Finally, artemisinin was identified by comparing the color intensity of the artemisinin standard to the other extracts [23].
The TLC analysis provided insight into the concentration of artemisinin in the standard and various root types evaluated. The artemisinin standard (1000 ppm) contained the highest artemisinin concentration since it was the darkest spot on the TLC plate. In comparing the color intensity of the hairy roots extract and the control roots extract on the TLC plate, it was determined that the hairy roots extract possessed a higher concentration of artemisinin than the control roots. In essence, this research confirmed that inducing the formation of hairy roots in the leaves of A. annua proved effective for the increased production of artemisinin.
High-performance liquid chromatography (HPLC) is a ubiquitous technique utilized for the isolation of secondary metabolites. Natural products such as secondary metabolites are typically isolated after the examination of the crude extract in a biological assay; this is performed to achieve a more holistic characterization of the bioactive constituents [22]. The meager quantities of bioactive compounds in the extract make the resolving power of HPLC critical for the swift processing of multi-component plant samples on a preparative and analytical scale [22]. Currently, numerous benchtop HPLC instruments possess a modular design and are inclusive of a solvent delivery pump, an auto-sampler or manual injection valve, a guard column, an analytical column, a detector, and a recorder or a printer [22]. HPLC can be employed for chemical separations because the constituents of the extract possess distinct migration rates and certain parameters are established. Ultimately, the mobile phase and stationary phase chosen determines the extent of separation [22]. An isocratic system (makes use of a single mobile phase system) is the general method utilized for the separation and identification of phytochemicals. However, if multiple sample components are of interest to the researcher, it would be ideal to employ gradient elution that involves the modification of the organic and aqueous proportions over a specified amount of time [22]. Gradient elution may prove challenging if the analytes possess analogous properties and similar retention times.
In HPLC, purification can be achieved by separating the compound of interest from other compounds or interferents. Based on the chromatographic conditions selected, each compound will possess a characteristic peak. To separate a variety of compounds, the chromatographer has the unique ability to tamper with parameters such as the mobile phase, column type, flow rate, and detectors to ascertain optimal peak resolution and separation [22]. For the identification of compounds, a suitable detector for HPLC must be chosen [22]. The chosen detector should be set to appropriate detection settings and a method is to be developed to yield a clean peak on the chromatogram for the specific analyte being analyzed [22]. UV detectors are quite commonplace among researchers because they afford high sensitivity [23]. Furthermore, phytochemicals of research interest commonly have a UV absorbance at low wavelengths that ranges from 190–210 nm [22]. Apart from UV detectors, other detectors such as the diode array detector (DAD) coupled with a mass spectrometer are employed to assess phytochemicals.
There have been several studies that have been successful in quantifying bioactive compounds present in plant material. The paramount purpose of the research performed by Sakipova et al. (2017) was to establish an efficient and distinct method for santonin identification and quantification with the use of HPLC [24]. The researchers more specifically desired to diminish unwarranted intoxication of santonin present in Artemisia cina so that its anthelmintic properties can be used in veterinary medicine [24]. The method developed from this research was utilized to characterize and quantify santonin levels in the leaves of eight distinct Artemisia species: A. cina, A. scoparia, A. absinthium, A. terra-albae, A. gmelinni, A. sublesingiana, A. schrenkiana, and A. frigida [24]. The primary result of this investigation demonstrated that santonin in Artemisia cina possessed a retention time was approximately 5.7 min, a time congruent with the Santonin standard tested [24].
The HPLC system (Waters) was employed for the quantitative analysis of santonin equipped with the breeze software program, Waters 717 plus autosampler, Waters 1525 binary HPLC pump, and Waters 2487 dual wavelength absorbance detector [24]. The solvent system applied was water (solvent A) and acetonitrile (solvent B). The gradient elution program utilized was: 35% A—65% B at 0 min, 35% A—65% B for 5 min, 45% A—55% B for 10 min, 55% A—45% B for 15 min, and 65% A—45% B for 20 min [24]. The chosen HPLC system parameters for the wavelength and pressure were 236 nm and 5000 atm, respectively [24]. HPLC chromatogram for chloroform extract from Artemisia cina is shown in Figure 3.
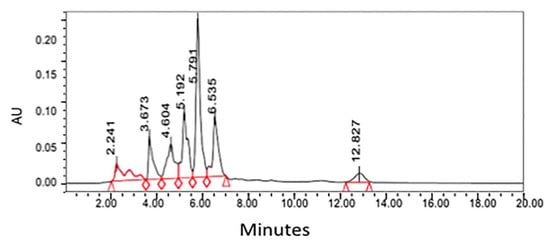
Figure 3. HPLC chromatogram for chloroform extract from Artemisia cina [24]. Peak at 5.7 represents santonin and quantified by the standard of santonin run with same HPLC condition.
In another investigation led by Tian et al. (2020), six phenolic acids derived from Artemisia capillaris (Yinchen) were analyzed using HPLC and their transformation pathways assessed during the decoction process [25]. The intent of this research was to establish and corroborate a novel analytical protocol for the analysis of phenolic acids such as 4,5-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, 1,3-dicaffeoylquinic acid, 4-caffeoylquinic acid, and 3-caffeoylquinic acid to achieve “quality control” of A. capillaris decoction [25]. To accomplish this purpose, HPLC coupled with diode array detection (HPLC-DAD) was employed [25].
The HPLC system utilized for the quantitative investigation of the six phenolic acids was a Thermo UltiMate 3000 (USA) containing an autosampler, a column temperature controller, and a DAD (190–300 nm) [25] is shown in Figure 4. To maintain the column temperature at 30 °C, an Agilent Eclipse XDB-C18 column (250 mm × 4.6 mm) with a particle size of 5 µm was used [25]. The applied solvent system was a 0.1% formic acid in water (solvent A) and acetonitrile (solvent B) [25]. The gradient elution program employed was: 7–15% B for 0–20 min (linear gradient), 15–20% B for 20–30 min (linear gradient), 20% B for 30–35 min (isocratic gradient), and 20–25% for 35–45 min (linear gradient) [25]. With an injection volume of 5 µL and a 5 min post-run at a flow rate of 1.0 mL/min, a high resolution of the desired compound peaks was achieved [25].
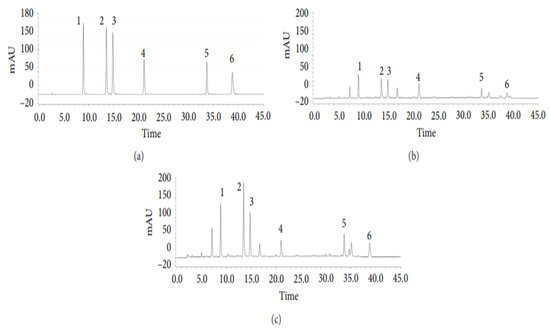
Figure 4. Representative HPLC chromatograms of (a) mixed standards; (b) A. capillaris herb; (c) A. capillaris decoction. Each numerical number signifies respective compounds: (1) 5-Caffeoylquinic acid; (2) 3-caffeoylquinic acid; (3) 4-caffeoylquinic acid; (4) 1,3-dicaffeoylquinic acid; (5) 3,4-dicaffeoylquinic acid; (6) 4,5-dicaffeoylquinic acid [25].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26226995
References
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228.
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109.
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566.
- Weston, L.; Barney, J.; DiTommaso, A. A Review of the Biology and Ecology of Three Invasive Perennials in New York State: Japanese Knotweed (Polygonum cuspidatum), Mugwort (Artemisia vulgaris) and Pale Swallowwort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69.
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a Better Understanding of Artemisia Vulgaris: Botany, Phytochemistry, Pharmacological and Biotechnological Potential. Food Res. Int. 2018, 109, 403–415.
- Available online: https://plants.sc.egov.usda.gov/home (accessed on 11 November 2020).
- Datasheet Report for Artemisia biennis (Biennial Wormwood). Available online: https://www.cabi.org/isc/datasheetreport/112441 (accessed on 19 June 2020).
- Mojarrab, M.; Mehrabi, M.; Ahmadi, F.; Hosseinzadeh, L. Protective Effects of Fractions from Artemisia Biennis Hydro- Ethanolic Extract against Doxorubicin-Induced Oxidative Stress and Apoptosis in PC12 Cells. Iran. J. Basic Med. Sci. 2016, 19, 8.
- Biennial Wormwood (Artemisia biennis)|Idaho Fish and Game. Available online: https://idfg.idaho.gov/species/taxa/45280 (accessed on 22 December 2020).
- Ivanescu, B.; Lungu, C.; Vlase, L.; Gheldiu, A.M.; Grigorescu, C.; Corciova, A. Bioactive Compounds from Artemisia campestris L. Subsp. Campestris. Rev. Chim. 2018, 69, 3076–3081.
- Dib, I.; El Alaoui-Faris, F.E. Artemisia Campestris L.: Review on Taxonomical Aspects, Cytogeography, Biological Activities and Bioactive Compounds. Biomed. Pharmacother. 2019, 109, 1884–1906.
- Setzer, W.N.; Vogler, B.; Schmidt, J.M.; Leahy, J.G.; Rives, R. Antimicrobial Activity of Artemisia Douglasiana Leaf Essential Oil. Fitoterapia 2004, 75, 192–200.
- Watson, L. CalPhotos: Artemisia douglasiana California Mugwort. Available online: https://calphotos.berkeley.edu/cgi/img_query? (accessed on 23 December 2020).
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (Tarragon): A Critical Review of Its Traditional Use, Chemical Composition, Pharmacology, and Safety. J. Agric. Food Chem. 2011, 59, 11367–11384.
- Aglarova, A.M.; Zilfikarov, I.N.; Severtseva, O.V. Biological Characteristics and Useful Properties of Tarragon (Artemisia dracunculus L.) (Review). Pharm. Chem. J. 2008, 42, 81–86.
- Artemisia dracunculus—Plant Finder. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=277563&isprofile=0& (accessed on 23 December 2020).
- Kelley, B.D.; Appelt, J.M.; Appelt, G.D. Artemisia Tridentata (Basin Sagebrush) in the Southwestern United States of America: Medicinal Uses and Pharmacologic Implications. Int. J. Addict. 1992, 27, 347–366.
- Gallego, R.; Montero, L.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Green Extraction of Bioactive Compounds from Microalgae. J. Anal. Test. 2018, 2, 109–123.
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents as Green and Efficient Media for the Extraction of Artemisinin from Artemisia Annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278.
- Mannan, A.; Ahmed, I.; Arshad, W.; Asim, M.F.; Qureshi, R.A.; Hussain, I.; Mirza, B. Survey of Artemisinin Production by Diverse Artemisia Species in Northern Pakistan. Malar. J. 2010, 9, 310.
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685.
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, Isolation and Characterization Of Bioactive Compounds From Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8.
- Zanousi, M.B.P.; Soleimani, T.; Keyhanfar, M.; Shirali, S.; Raeesi, M. Thin Layer Chromatography (TLC) Technique in the Investigation of Artemisinin Production in Artemisia annua L. Medicinal Plant Hairy Roots. J. Med. Plants Res. 2012, 6, 1842–1845.
- Sakipova, Z.; Wong, N.S.H.; Bekezhanova, T.; Sadykova Shukirbekova, A.; Boylan, F. Quantification of Santonin in Eight Species of Artemisia from Kazakhstan by Means of HPLC-UV: Method Development and Validation. PLoS ONE 2017, 12, e0173714.
- Tian, F.; Ruan, Q.-J.; Zhang, Y.; Cao, H.; Ma, Z.-G.; Zhou, G.-L.; Wu, M.-H. Quantitative Analysis of Six Phenolic Acids in Artemisia capillaris (Yinchen) by HPLC-DAD and Their Transformation Pathways in Decoction Preparation Process. Available online: https://www.hindawi.com/journals/jamc/2020/8950324/ (accessed on 13 July 2020).
This entry is offline, you can click here to edit this entry!
