UiO-66-NH2 is a metal–organic framework (MOF), which is constructed of zirconium and amino-terephthalate ions. Modification of MOFs with photochromic compounds allows managing their gas capacity and directing sorption-desorption processes. Photochromic molecules are able to reverse their configuration under UV‐light irradiation affecting available pore volume. The modification of UiO‐66‐NH2 with diarylethene molecules (DAE, 4‐(5‐Methoxy‐1,2‐dimethyl‐1H‐indol‐3‐yl)‐3‐(2,5‐dimethylthiophen‐3‐yl)‐4‐furan‐2,5‐dione) results in the formation of new photoswitchable material for light-driven H2 storage. Most of the DAE molecules inside of the UiO‐66‐pores had an open conformation after synthesis. However, the equilibrium was able to be shifted further toward an open conformation using visible light irradiation with a wavelength of 520 nm. Conversely, UV‐light with a wavelength of 450 nm initiated the transformation of the photoresponsive moieties inside of the pores to a closed modification. We have shown that this transformation could be used to stimulate hydrogen adsorption–desorption processes. Specifically, visible light irradiation increased the H2 capacity of modified MOF, while UV‐light decreased it. A similar hybrid material with DAE moieties in the UiO‐66 scaffold was applied for hydrogen storage for the first time. Additionally, the obtained results are promising for smart H2 storage that is able to be managed via light stimuli.
- metal–organic frameworks
- secondary building units
- UiO-66
1. Introduction

2. Synthesis

Figure 2. Scheme of UiO‐66‐NH2 functionalization.
The modification of UiO‐66‐NH2 was performed according to previously reported methods, with some modifications that were made based on the specific objective of obtaining diarylethenes with pyrrole‐2,5‐dione bridging fragments [35–37]. A solution containing 0.36 mmol of DAE in isopropyl alcohol (30 mL) was treated with UiO‐66‐NH2 (0.06 mmol) and 4‐dimethylaminopyridine (DMAP) (1 mg). The reaction mixture was refluxed for 10 h and cooled, and the solvent was removed by means of distillation. The resulting precipitate was washed with CH2Cl2 until no staining was detected. The obtained sample was designated as DAE‐UiO‐66.
3. Results
Both of the UiO‐66‐NH2 and DAEUiO‐66 samples had cubic symmetry with the Fm‐3m (225) space group. The lattice constants increased from 20.794 Å (UiO‐66‐NH2) to 20.836 Å (DAE‐UiO‐66). This could indicate stress in the framework of the DAE‐UiO‐66 sample due to the pores becoming filled with relatively photochromic molecules. The DAE‐UiO‐66 sample consisted of octahedral crystals that were 60–90 nm in size (Figure 3). In good agreement with the XRD data, we observed that the DAE‐UiO‐66 sample demonstrates lower porosity compared to the initial sample (Table 1).

Figure 3. (a) XRD profiles of the UiO‐66‐NH2 (black) and DAE‐UiO‐66 (brown) samples. Intensities are normalized and shifted along the y‐axis for better representation. (b) SEM image of DAR‐UiO‐66 sample.
Table 1. Some properties of the synthesized samples UiO‐66‐NH2 and DAE‐UiO‐66. SSA stands for the specific surface
area.
| Sampes' designation | Unit cell parameter a=b=c, Å | SSA, m2/g | DAE content from TGA, mol.% | DAE content from XRF, mol.% |
| UiO‐66‐NH2 | 20.794(5) | 1111 | - | - |
| DAE‐UiO‐66 | 20.836(6) | 398 | 9 | 8 |
Analyzing joint data of TGA and XRF, we proposed the following chemical composition of DAE‐UiO‐66:
Zr6O4(OH)4(C8H5O4N)5.2(C8H6O4NCl)0.3(C29H23O7N3S)0.5. One octahedral pore of UiO-66-NH2 contains one DAE molecule.
We have traced the hydrogen capacity for the DAE‐UiO‐66 sample that was consistently irradiated with visible and UV light. The isotherms are presented in Figure 4a. After irradiation with visible light (wavelength 520 nm), the as‐synthesized sample adsorbed 0.58 weight% of hydrogen at 77 K. This value is slightly higher than the H2 capacity for the as‐synthesized sample (0.55 weight%). Irradiation with UV‐light (450 nm) reduced the hydrogen capacity. These changes are reversible because the same trend was reproduced after further irradiations with visible and UV light (Figure 4b).
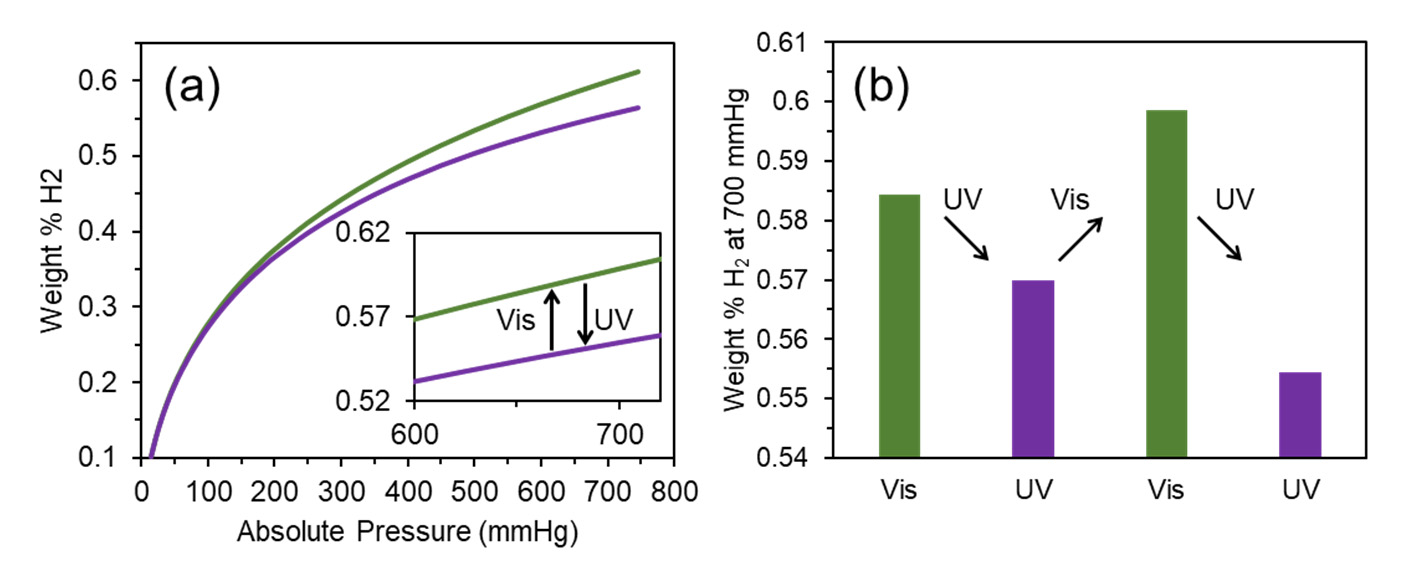
Figure 4. (a) Isotherms of hydrogen adsorption (77 K) for DAE‐UiO‐66 sample irradiated with visible light with a wavelength of 520 nm (green plot) and UV‐light with a wavelength of 450 nm (violet plot). (b) Diagram of changing in H2 capacity during sequential irradiation with visible light with a wavelength of 520 nm (green columns) and UV‐light with a wavelength of 450 nm (violet columns).
4. Discussion
According to the experimental data, we propose that the DAE molecules undergo the following transformations inside of the pores (Figure 5). After the synthesis, most of the DAE molecules are in the “open” modification, as confirmed by UV–Vis spectra. After irradiation with visible light, this equilibrium strongly shifts to the left, resulting in the open form being the dominant DAE modification. The open form increased hydrogen capacity and resulted in pronounced changes in the IR spectrum. Under UV‐irradiation, the DAE molecules underwent a “closed” modification, which was determined according to
UV–Vis spectroscopy. Additionally, this resulted in the DAE‐UiO‐66 sample having a lower H2 capacity.

Figure 5. Scheme of DAE transformations inside DAE‐UiO‐66 pores under UV‐light (hν1) and visible light (hν2).
5. Conclusions
In summary, we modified UiO‐66‐NH2 with the photoactive DAE molecule 4‐(5‐Methoxy‐1,2‐dimethyl‐1H‐indol‐3‐yl)‐3‐(2,5‐dimethylthiophen‐3‐yl)‐4‐furan‐2,5‐dione. The obtained material was highly crystalline and was isostructural to the initial UiO‐66‐NH2 scaffold. An increase in the lattice constants was caused by stress due to the molecules in the pores. The obtained material, DAE‐UiO‐66, demonstrated a preserved porous structure with SSA of almost 400 m2/g. According to a complex analysis of the IR‐spectra, UV–Vis spectra, and hydrogen adsorption isotherms, we can conclude that the as‐modified sample contained a mixture of two forms of DAE‐molecules—”open” and “closed”. The equilibrium was shifted to the “open” modification from the very beginning. However, some changes could be observed after the irradiation of DAE‐UiO‐66 with a visible light source, initiating the transformation of the DAE fillers in the pores to the open conformation. This increased the hydrogen capacity. We measured the hydrogen capacity under UV/Vis irradiation and observed a reversible process that was able to be managed via light stimuli. Specifically, visible light increases the H2 capacity, while UV‐irradiation decreases it. We suppose that this result is an important step in the development of smart hydrogen storage with light‐induced safe H2‐desorption.
This entry is adapted from the peer-reviewed paper 10.3390/polym13224052
References
- Yaghi, O.M.; OʹKeeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J.; Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714, doi:10.1038/nature01650.
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C.; Metal‐organic frameworks: Structure, properties, methods of synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307, doi:10.1070/rcr4554.
- Gui, B.; Meng, Y.; Xie, Y.; Du, K.; Sue, A.C.H.; Wang, C.; Immobilizing Organic‐Based Molecular Switches into Metal‐Organic Frameworks: A Promising Strategy for Switching in Solid State. Macromol. Rapid Commun. 2018, 39, 1700388, 10.1002/marc.201700388.
- Huang, S.L.; Hor, T.S.A.; Jin, G.X.; Photodriven single‐crystal‐to‐single‐crystal transformation. Coord. Chem. Rev. 2017, 346, 112– 122, 10.1016/j.ccr.2016.06.009.
- Rice, A.M.; Martin, C.R.; Galitskiy, V.A.; Berseneva, A.A.; Leith, G.A.; Shustova, N.B.; Photophysics Modulation in Photoswitchable Metal‐Organic Frameworks. Chem. Rev. 2020, 120, 8790–8813, 10.1021/acs.chemrev.9b00350.
- Luo, F.; Fan, C.B.; Luo, M.B.; Wu, X.L.; Zhu, Y.; Pu, S.Z.; Xu, W.Y.; Guo, G.C.; Photoswitching CO2 Capture and Release in a Photochromic Diarylethene Metal‐Organic Framework. Angew. Chem.‐Int. Edit. 2014, 53, 9298–9301, 10.1002/anie.201311124.
- Gong, L.L.; Feng, X.F.; Luo, F.; Novel azo‐Metal‐Organic Framework Showing a 10‐Connected bct Net, Breathing Behavior, and Unique Photoswitching Behavior toward CO2. . Inorg. Chem. 2015, 54, 11587–11589, 10.1021/acs.inorgchem.5b02037.
- Cox, J.M.; Walton, I.M.; Benedict, J.B.; On the design of atropisomer‐separable photochromic diarylethene‐based metal‐organic framework linkers.. J. Mater. Chem. C 2016, 4, 4028–4033, 10.1039/c6tc00131a.
- Fan, C.B.; Gong, L.L.; Huang, L.; Luo, F.; Krishna, R.; Yi, X.F.; Zheng, A.M.; Zhang, L.; Pu, S.Z.; Feng, X.F.; et al. Significant Enhancement of C2H2/C2H4 Separation by a Photochromic Diarylethene Unit: A Temperature‐ and Light‐Responsive Separation Switch.. Angew. Chem.‐Int. Edit. 2017, 56, 7900–7906, 10.1002/anie.201702484.
- Furlong, B.J.; Katz, M.J.; Bistable Dithienylethene‐Based Metal‐Organic Framework Illustrating Optically Induced Changes in Chemical Separations.. J. Am. Chem. Soc. 2017, 139, 13280–13283, 10.1021/jacs.7b07856.
- Williams, D.E.; Rietman, J.A.; Maier, J.M.; Tan, R.; Greytak, A.B.; Smith, M.D.; Krause, J.A.; Shustova, N.B.; Energy Transfer on Demand: Photoswitch‐Directed Behavior of Metal‐Porphyrin Frameworks. J. Am. Chem. Soc. 2014, 136, 11886–11889, 10.1021/ja505589d.
- Park, J.; Feng, D.W.; Yuan, S.; Zhou, H.C.; Photochromic Metal‐Organic Frameworks: Reversible Control of Singlet Oxygen Generation. . Angew. Chem.‐Int. Edit. 2015, 54, 430, 10.1002/anie.201408862.
- Butova, V.V.; Burachevskaya, O.A.; Muratidi, M.A.; Surzhikova, I.I.; Zolotukhin, P.V.; Medvedev, P.V.; Gorban, I.E.; Kuzharov, A.A.; Soldatov, M.A.; Loading of the Model Amino Acid Leucine in UiO‐66 and UiO‐66‐NH2: Optimization of Metal‐Organic Framework Carriers and Evaluation of Host‐Guest Interactions.. Inorg. Chem. 2021, 60, 5694–5703, 10.1021/acs.inorgchem. 0c03751.
- Shepelenko, E.N.; Makarova, N.I.; Karamov, O.G.; Dubonosov, A.D.; Podshibakin, V.A.; Metelitsa, A.V.; Brenʹ, V.A.; Minkin, V.I.; Synthesis and Photochromic Properties of Asymmetric Dihetarylethenes Based on 5‐methoxy‐1,2‐dimethylindole and 5‐(4‐ bromophenyl)‐2‐methylthiophene.. Chem. Heterocycl. Compd. 2014, 50, 932–940, 10.1007/s10593‐014‐1547‐7.
