Gastric hyperplastic polyps (GHP) are frequently found to be benign polyps and have been considered to have a low carcinogenic potential. The characteristics of the hyperplastic polyp-associated gastric cancer (HPAGC) remain unclear.
1. Introduction
Gastric cancer is currently the third most common cause of cancer-related death in Japan
[1]. Inflammation is a key feature of gastric cancer, and oxidative stress due to chronic inflammation caused by
Helicobacter pylori plays an important role in the carcinogenesis of gastric cancer
[2]. Chronic atrophic gastritis and intestinal epithelialization are known to be precancerous conditions caused by
H. pylori [3]. In our previous study, we showed that the activation of oxidative stress and v-akt murine thymoma viral oncogene homolog (AKT) increased substantially in each of the following categories of chronic gastritis: chronic gastritis without
H. pylori, chronic active gastritis with
H. pylori, chronic metaplastic gastritis without
H. pylori, and chronic gastritis with atypia without
H. pylori [4]. This is due to the shortening of telomeres and activation of telomerase reverse transcriptase, which are associated with repeated mucosal regeneration due to chronic inflammation
[5] and are thought to lead to hyperplasia of gastric mucosal stem cells
[6][7].
Gastric hyperplastic polyps (GHPs) are the most common polyps encountered in the stomach (along with fundic gland polyps) and are detected in 1.9% of 110,000 patients subjected to gastroscopy
[8]. Patients with GHPs are usually asymptomatic; however, some may present with dyspepsia, heartburn, abdominal pain, anemia, or upper gastrointestinal bleeding
[9]. GHPs are thought to occur during the repair of damaged mucosa
[9]. Malignant transformation of GHP is very infrequent, with an incidence of 0.3–3%
[10][11]. In previous research, hyperplastic polyp-associated gastric cancer (HPAGC) is derived from dysplastic foci in GHP
[12][13]. However, the histological and molecular pathological markers for its definitive diagnosis have not been established.
2. GHP Atypia and the Associated Clinical and Morphological Characteristics
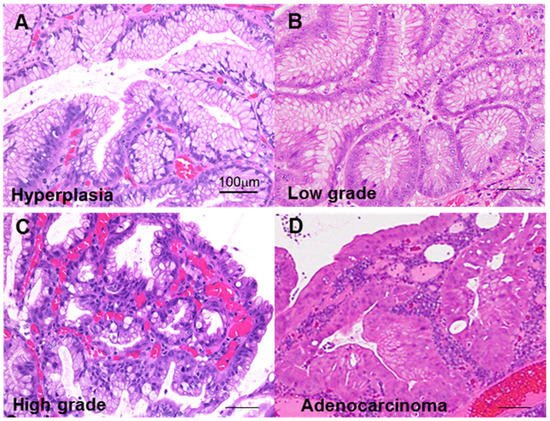
First, we classified GHP atypia into four categories: non-atypical GHP with no nuclear and no structural atypia with abundant mucus production; low-grade atypia with mild to moderate nuclear swelling; high-grade atypia with moderate to prominent nuclear swelling, pseudostratified nuclei, and decreased periodic acid–Schiff (PAS)-positive mucus production; and HPAGC with the cellular characteristics of high-grade atypia as well as structural atypia and/or invasion (Figure 1).
Figure 1. Atypia in hyperplastic polyps. (A) Hyperplastic polyp without atypia. (B) Low-grade atypia, notable mild nuclear swelling. (C) High-grade atypia, notable moderate to marked nuclear swelling, nuclear pseudostratification, and decrease in mucus production. (D) Carcinoma in hyperplastic polyp (well-differentiated tubular adenocarcinoma), marked nuclear swelling, nuclear pseudostratification, decrease in mucus production, and structural atypia. Scale bar, 100 μm.
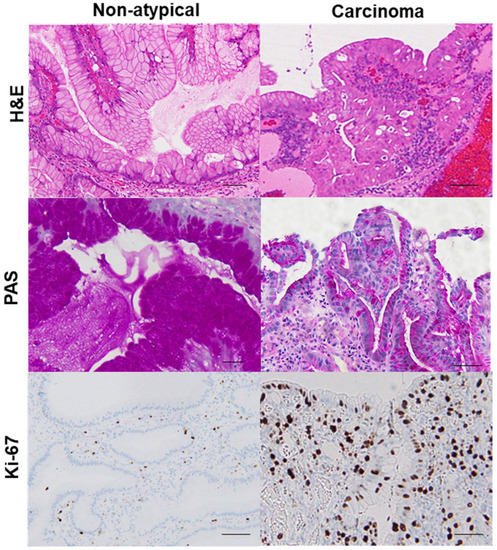
Using this categorization process to sort the samples from 102 GHP patients, we identified 20 GHP samples as low-grade atypia, 7 GHP samples as high-grade atypia, and 5 GHP samples as HPAGC (Table 1). Atypical GPHs were more common in the elderly and the incidence increased with an increase in polyp size. In particular, polyps larger than 1 cm were associated with a higher grade and cancer. In addition, mucus production decreased with increasing atypia. By contrast, no correlation was observed between atypia and H. pylori infection or intestinal metaplasia. Immunohistochemistry for Ki-67 expression showed that enhanced proliferative activity also correlated with atypia (Figure 2).
Figure 2. Comparison of mucus production and proliferative activity between a hyperplastic polyp and its carcinoma lesion.In the same hyperplastic polyp (case 2 in Table 3), non-atypical regions and the carcinoma lesion were compared. In the non-atypical region, PAS-positive mucus is abundant in most foveolar epithelial cells, whereas in the cancer lesion, PAS-positive mucus is only found in a few cells. Ki-67-positive cells were scattered in non-atypical regions with an index of 5%. By contrast, in the cancer lesion, a high number of Ki-67-positive cells were evident, up to the surface layer of the glands (index of 92%). H&E, hematoxylin and eosin; PAS, periodic acid–Schiff.
Table 1. Relationship between polyp atypia and H. pylori infection or proliferation.
| Parameter |
Polyp Atypia (1) |
p-Value |
| None |
Low Grade |
High Grade |
Cancer |
| Number |
72 |
20 |
7 |
5 |
|
| Age (yrs) |
55 ± 13 |
60 ± 11 |
63 ± 10 |
70 ± 5 |
0.0191 |
| Sex (male: female) |
22:22 |
12:8 |
4:3 |
3:2 |
NS |
| Size (mm) |
4.7 ± 1.8 |
6.9 ± 1.7 |
12.3 ± 5.9 |
14.6 ± 5.4 |
<0.0001 |
| H. pylori infection |
|
|
|
|
|
| Incidence (%) |
9 |
10 |
0 |
20 |
NS |
| Grade (2) |
0.18 ± 0.65 |
0.10 ± 0.31 |
0 |
0.20 ± 0.45 |
NS |
| Intestinal metaplasia (2) |
|
|
|
|
|
| Incidence (%) |
32 |
55 |
29 |
40 |
NS |
| Grade (2) |
0.5 ± 0.88 |
0.55 ± 0.51 |
0.29 ± 0.49 |
0.40 ± 0.55 |
NS |
| PAS staining (3) |
2.0 ± 0.1 |
1.4 ± 0.5 |
0.8 ± 0.3 |
0.6 ± 0.2 |
<0.0001 |
| Ki-67 index (%) (4) |
26 ± 13 |
52 ± 23 |
74 ± 18 |
86 ± 10 |
<0.0001 |
(1) Low grade, mild to moderate nuclear swelling; high grade, moderate to marked nuclear swelling, nuclear pseudostratification, and weakened mucus production; cancer, structural atypia, and/or invasion in addition to alterations similar to high-grade lesions. (2) According to the updated Sydney classification [14], each grade (none, mild, moderate, and severe) was quantified as 0, 1, 2, and 3, respectively, and the results were statistically analyzed. (3) PAS staining grades were classified according to the ratio of the area of the PAS-positive region in the cells: 0, 0%; 0.5, <10%; 1, 10–25%; 2, 25–50%; 3, >50%. (4) Ki-67 staining was examined in 1000 epithelial cells and the frequency of positive nuclear staining was determined.
3. GHP Atypia and Oxidative Stress
Oxidative stress has a prominent influence on gastric carcinogenesis
[2]. When we investigated the relationship between atypia and oxidative stress in GHPs (
Figure 3,
Table 2), we found a correlation between atypia and nuclear 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels. Interestingly, 4-hydroxy nonenal (HNE) levels in the granulation tissue of GHPs also correlated with atypia, as did the area ratio of granulation tissue within the polyps. Nuclear 8-OHdG levels and granulation tissue area, or 4-HNE levels in granulation tissue, were all correlated (Pearson r = 0.6489 and 0.7245,
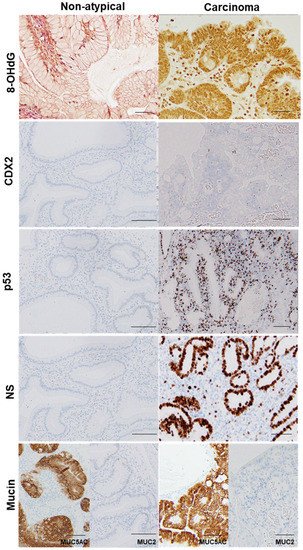
p < 0.0001 and < 0.0001, respectively). These results suggest that granulation tissue may play a role in generating oxidative stress in GHP, and it may be a trigger for GHP carcinogenesis.
Figure 3. Comparison of oxidative stress, intestinal phenotype, and stemness between hyperplastic polyp and its carcinoma lesion.
Table 2. Relationship between atypia and granulation tissue, oxidative stress, or stemness.
| Parameter |
Polyp Atypia (1) |
p-Value |
| None |
Low Grade |
High Grade |
Cancer |
| Number |
44 |
20 |
7 |
5 |
|
| Granulation tissue (%) (2) |
15 ± 12 |
48 ± 23 |
79 ± 7 |
87 ± 7 |
<0.0001 |
| Tumor 8-OHdG index (3) |
23 ± 12 |
52 ± 18 |
86 ± 8 |
94 ± 5 |
<0.0001 |
| 4-HNE (ng/g) (4) |
0.5 ± 0.1 |
0.8 ± 0.2 |
1.8 ± 0.3 |
2.4 ± 0.3 |
<0.0001 |
| CDX2 incidence (%) (3) |
25 |
50 |
29 |
40 |
NS |
| p53 incidence (%) (3) |
0 |
0 |
43 |
100 |
0.0075 |
| NS index (%) (3) |
18 ± 8 |
53 ± 17 |
80 ± 2 |
84 ± 6 |
<0.0001 |
(1) Low grade, mild to moderate nuclear swelling; high grade, moderate to marked nuclear swelling, nuclear pseudostratification, and weakened mucus production; cancer, structural atypia, and/or invasion in addition to alterations similar to high-grade lesions. (2) Evaluated according to the area occupied by the polyp: 0, <10%; 1, 10–50%; 2, 50–75%; 3, >75%. (3) For assessment of these parameters, 1000 epithelial cells were examined, and the frequency of positive nuclear staining was determined. For p53 and CDX2, cases were judged as positive when the frequency of positive cells (index) was 10% or more. (4) 4-HNE levels in extracts from granulation tissues were measured using ELISA.
In the same hyperplastic polyp (case 2 in Table 3), non-atypical regions and the carcinoma lesion were compared. 8-OHdG in the nuclei was scattered in non-atypical regions (index of 4%), whereas the cancer lesions exhibited a substantial number of positive cells (index of 100%). Both non-atypical regions and the cancer lesion showed no nuclear expression of CDX2. p53 nuclear accumulation was found in the cancer lesion (index of 72%), but not in non-atypical regions (index of 0%). Nuclear staining for NS was only observed in a few cells in non-atypical regions (index of less than 1%), whereas cancer lesions showed high labeling of NS (index of 87%). 8-OHdG, 8-hydroxy-2′-deoxyguanosine; CDX2, caudal type homeobox transcription factor 2; NS, nucleostemin.
Table 3. Adenocarcinoma cases in hyperplastic polyps.
| |
Case |
| 1 |
2 |
3 |
4 |
5 |
| Polyp size (mm) |
11 |
8 |
20 |
20 |
14 |
| Cancer lesion (mm) |
2 |
4 |
3 |
3 |
2 |
| Histology (1) |
tub1 |
Pap + tub1 |
tub1 |
tub1 |
Pap + tub1 |
| Invasion |
In situ |
In situ |
Invasive |
In situ |
In situ |
| Mucin type (2) |
Mixed |
Gastric |
Gastric |
Mixed |
Mixed |
| Ki-67 index (%) |
94 |
92 |
85 |
70 |
90 |
| 8-OHdG index (%) (3) |
100 |
100 |
92 |
88 |
90 |
| CDX2 index (%) (3) |
24 |
0 |
0 |
5 |
80 |
| p53 index (%) (3) |
78 |
72 |
19 |
17 |
89 |
| NS index (%) (3) |
78 |
87 |
85 |
82 |
88 |
| KRAS G12D/G13D |
-/- |
-/- |
-/- |
-/- |
-/- |
| BRAF V600E |
- |
+ |
+ |
- |
- |
(1) Histological classification was based on the Japanese Gastric Cancer Classification guidelines [15]. Pap, papillary adenocarcinoma; tub1, well-differentiated tubular adenocarcinoma. (2) Mixed, MUC5AC+/MUC2+; gastric, MUC5AC+/MUC2-. (3) These parameters were determined by examining 1000 epithelial cells and recording the frequency of positive nuclear staining.
4. GHP Atypia and Expression of Cancer-Associated Proteins
We next examined atypia in GHP samples and the expression of cancer-associated proteins, namely caudal type homeobox transcription factor 2 (CDX2), p53, and nucleostemin (NS) (
Figure 3,
Table 2). CDX2, which is associated with the acquisition of the intestinal phenotype associated with
H. pylori infection
[16], did not correlate with atypia (similarly to the results for
H. pylori and intestinal metaplasia). By contrast, p53 expression was negative in non-atypical GHPs and GHPs with low-grade atypia, but positive for 43% of high-grade GHP samples and 100% positive for the HPAGC samples. In addition, the number of cells that were positive for NS (a stem cell marker) increased in correlation with atypia.
5. Features of HPAGC
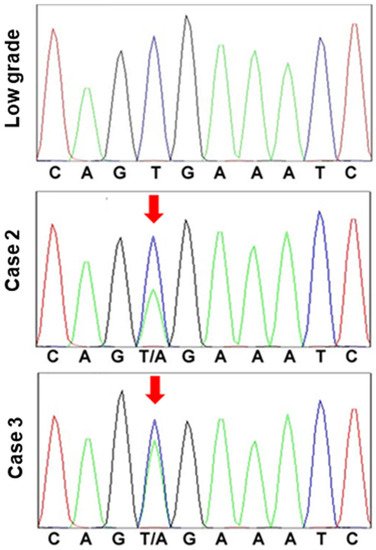
Finally, we examined the five cases in which cancer was found in the polyps (Table 3). In these cases, the size of the polyp exceeded 1 cm in four of the five cases. All cancer lesions were 4 mm or less intramucosal cancers with a well-differentiated histology and were carcinomas in situ, except for one case. Two cases exhibited CDX2-positive staining in ≥10% of the cells. One case had CDX2-positive cells in 5% of the cells, whereas the other two were negative for CDX2. Each of the three CDX2-positive cases was also characterized by a mixed mucin phenotype, whereas the two CDX2-negative cases exhibited a gastric mucin phenotype. There was no correlation between polyp size and p53 or NS indices, histology, CDX2 expression, or mucin type; and all of the cases were negative for KRAS G12D/G13D mutations. By contrast, BRAF V600E mutation was found in the two cases which exhibited a gastric mucin phenotype (Figure 4).
Figure 4. BRAF mutation in hyperplastic polyp carcinoma.
BRAF exon 15 was sequenced in a low-grade atypia polyp and hyperplastic polyp-associated gastric cancer (cases 2 and 3). In the low-grade atypia polyp, codon 600 exhibited the normal sequence, G-T-G (Val). By contrast, in the cancer cases, codon 600 was determined to be mutated, with the sequence G-T/A-G (Val/Glu).
This entry is adapted from the peer-reviewed paper 10.3390/ijms222312724