The successful establishment of many plants in tropical forests often depends on species-specific adaptations related to light availability and forest successional stage. Species that are present in early successional stages generally do not occur in later successional stages.
- light availability
- establishment success
- invasive plant species
1. Introduction
2. Forest Edge Effects on Distribution and Abundance of Terrestrial Invasive Herbs and Shrubs
3. Species Diversity and Distribution of Terrestrial Invasive Herbs and Shrubs in Hainan Island
| Habitat (N) | Ap | Co | Cs | Lc | Mp | Pc | Pf | Ph | St | Species Per Site (Mean ± Stdev) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abandoned field (17) | + | + | + | + | + | + | + | + | + | 4.6 ± 1.3 |
| Plantation (20) | 0 | + | + | + | + | + | 0 | 0 | + | 4.2 ± 1.0 |
| Rural (14) | + | + | + | + | + | + | + | + | + | 3.9 ±0.8 |
| Grassland (11) | + | + | + | + | + | + | 0 | 0 | + | 3.4 ± 0.9 |
| Field (14) | + | + | + | + | + | + | + | + | + | 3.6 ± 0.8 |
| Forest edge (11) | 0 | + | + | + | + | + | 0 | 0 | + | 2.7 ± 1.3 |
| Rain forest (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 ± 0.0 |
| TIHS | Frequency of Terrestrial Invasive Herbs and Shrubs | |||||
|---|---|---|---|---|---|---|
| AF (17) | PL (20) | RV (14) | SE (11) | FI (14) | GR (11) | |
| Pc | 0.700 ± 0.500 a | 0.946 ± 0.472 a | 0.485 ± 0.298 b | 0.556 ± 0.529 b,c | 0.790 ± 0.451 b,c | 0.685 ± 0.218 b |
| Mp | 0.502 ± 0.407 a | 0.331 ± 0.357 a | 0.274 ± 0.351 a,b | 0.204 ± 0.430 a | 0.592 ± 0.455 c | 0.474 ± 0.250 a,b |
| Co | 0.749 ± 0.493 a | 0.701 ± 0.465 a | 0.628 ± 0.326 a | 0.556 ± 0.529 a | 0.733 ± 0.459 a | 0.771 ± 0.226 a |
| Cs | 0.273 ± 0.431 a | 0.339 ± 0.443 a | 0.291 ± 0.394 a | 0.186 ± 0.347 a | 0.355 ± 0.527 a | 0.191 ± 0.094 a |
| PF | 0.118 ± 0.223 a | - | 0.112 ± 0.185 a | - | 0.051 ± 0.125 a | - |
| Lc | 0.118 ± 0.311 a | 0.025 ± 0.092 a | 0.112 ± 0.185 a | 0.099 ± 0.188 a | 0.051 ± 0.125 a | 0.092 ± 0.137 a |
| Ap | 0.126 ± 0.211 a | 0.022 ± 0.115 a | 0.032 ± 0.105 a | - | 0.091 ± 0.114 a | 0.052 ± 0.137 a |
| St | 0.126 ± 0.391 a | 0.226 ± 0.173 b | 0.029 ± 0.140 a | 0.050 ± 0.169 a | 0.179 ± 0.397 a | 0.129 ± 0.140 a |
| Ph | 0.085 ± 0.121 a | 0.015 ± 0.002 a | 0.112 ± 0.105 a | - | 0.051 ± 0.125 a | 0.092 ± 0.037 a |
| AFTIHS ** | 2.806 | 2.605 | 2.075 | 1.651 | 2.893 | 2.486 |
* Different lowercase letters indicate significant differences between invaded vegetation types (p < 0.05). ** AFTIHS: Accumulated frequency of invasive plants. AF: abandoned field, PL: plantation, RV: rural vegetation, SE: shurb and edge of tropical forest, FI: field, GR: grassland. Ap: A. philoxeroides, Co: C. odorata, Cs: C. sumatrensis, Lc: L. camara, Mp: M. pudica, Pc: P. clematidea, PF: P. fasciculare, Ph: P. hysterophorus and St: S. trilobata.
4. Forest Edge Effects on Terrestrial Invasive Herbs or Shrubs
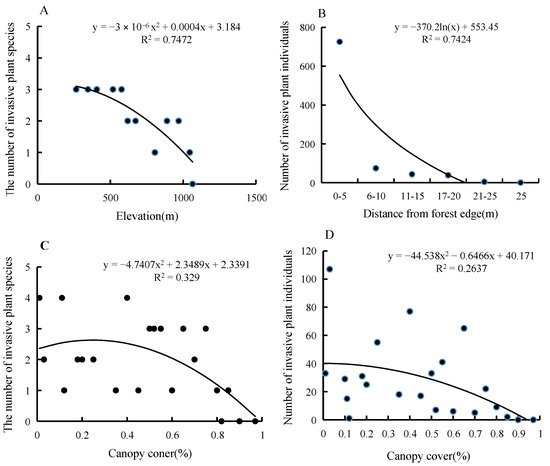
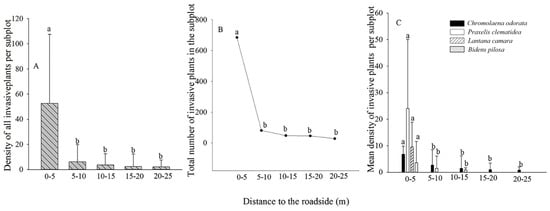
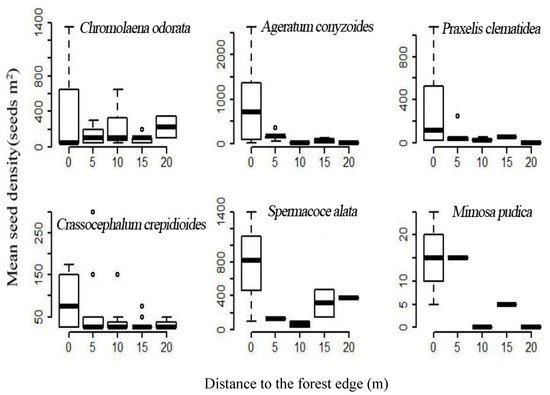
| Species | Frequency (Presence/Soil Sample) | Furthest Distance Observed (m) |
Range (Seedlings m−2) |
Density (Seedlings m−2) |
Total Germinated Seedlings of TIHS | Proportion of All Germinated Seedlings (57,138) (%) |
|---|---|---|---|---|---|---|
| A. conyzoides | 0.138 | 20 | 25–625 | 501.9 ± 697.0 | 542 | 0.95 |
| P. clematidea | 0.159 | 20 | 25–7000 | 192.5 ± 267.0 | 511 | 0.89 |
| B. latifolia | 0.041 | 20 | 25–1400 | 387.5 ± 483.8 | 143 | 0.25 |
| C. odorata | 0.149 | 20 | 25–2900 | 104.5 ± 139.0 | 117 | 0.2 |
| R. repen | 0.015 | 0 | 225–1375 | 783.3 ± 575.0 | 94 | 0.16 |
| C. crepidioide | 0.185 | 20 | 25–300 | 54.9 ± 59.7 | 79 | 0.14 |
| M. diplotricha | 0.015 | 10 | 25–275 | 166.5 ± 128.0 | 20 | 0.04 |
| M. pudica | 0.036 | 15 | 25–125 | 67.9 ± 37.4 | 19 | 0.03 |
| S. jamaicensis | 0.010 | 20 | 25–175 | 100.0 ± 106.1 | 8 | 0.01 |
| B. pilosa | 0.015 | 15 | 25–75 | 41.6 ± 28.8 | 5 | 0.01 |
| L. camara | 0.021 | 0 | 25 | 25.0 | 4 | 0.01 |
| T. procumbens | 0.015 | 10 | 25 | 25.0 | 3 | 0.01 |
| Total | 1545 | 2.7 |
| Effects | Parameter | Estimate (±1 SE) |
|---|---|---|
| (A) Pooled species richness of exotic species ~ Distance from edge + Canopy | Intercept | 1.114 (0.212) |
| Distance from the edge | −0.780 (0.383) | |
| Canopy cover | −0.796 (0.453) | |
| (B) Pooled abundance of exotic seeds ~Distance from edge + Canopy cover + seed mass | Intercept | 3.455 (0.302) |
| Distance from the edge | −4.291 (0.650) | |
| seed mass | −2.151 (0.715) | |
| Canopy cover | −0.526 (0.361) | |
| (C) Presence of exotic plants in the soil seed bank ~Distance from edge + Canopy cover | Intercept | 6.910 (2.867) |
| Distance from the edge | −2.627 (0.741) | |
| Canopy cover | 0.265 (0.820) |
5. Seed Germination and Seedling Survival of Terrestrial Invasive Herbs and Shrubs in Forest Gaps
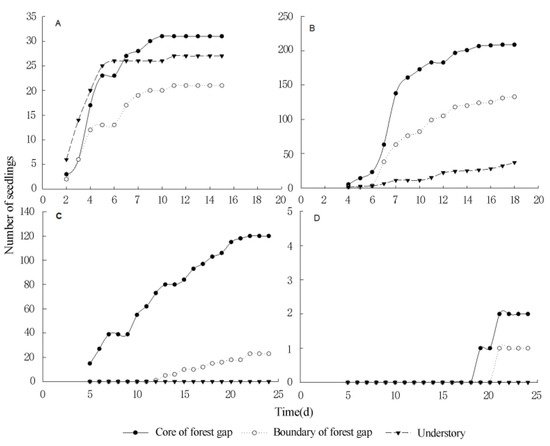
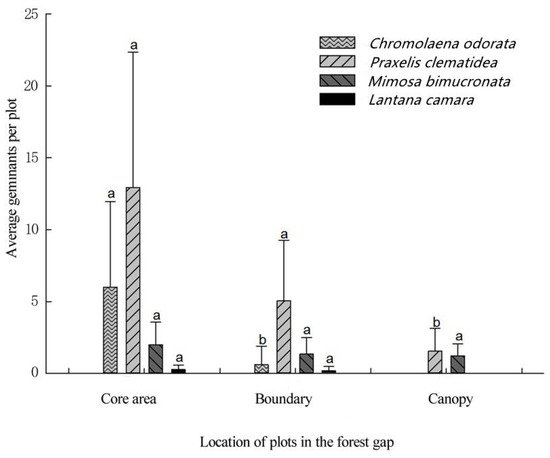
This entry is adapted from the peer-reviewed paper 10.3390/f12111596
References
- Peerbhay, K.; Mutanga, O.; Ismail, R. The identification and remote detection of alien invasive plants in commercial forests: An Overview. S. Afr. J. Geomat. 2016, 5, 49–67.
- Walsh, J.R.; Carpenter, S.R.; Zanden, V.M.J. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc. Natl. Acad. Sci. USA 2016, 113, 4081–4085.
- Gozlan, R.E.; Newton, A.C. Biological invasions: Benefits versus risks. Science 2009, 324, 1015–1016.
- Meisner, A.; Boer, W.E.; Verhoeven, K.J.F.; Boschker, H.T.S.; Putten, W.H.V.D. Comparison of nutrient acquisition in exotic plant species and congeneric natives. J. Ecol. 2011, 99, 1308–1315.
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Chapter One—Impacts of Invasive Species on Food Webs: A Review of Empirical Data. Adv. Ecol. Res. 2017, 56, 1–60.
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69.
- Prescott, C.E.; Zukswert, J.M. Invasive plant species and litter decomposition: Time to challenge assumptions. New Phytol. 2016, 209, 5–7.
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. Chapter Two—The Effects of Invasive Species on the Decline in Species Richness: A Global Meta-Analysis. Adv. Ecol. Res. 2017, 56, 61–83.
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170.
- Strauss, S.Y.; Webb, C.O.; Salamin, N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. USA 2006, 103, 5841–5845.
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709.
- Catford, J.A.; Baumgartner, J.B.; Vesk, P.A.; White, M.; Buckley, Y.M.; McCarthy, M.A. Disentangling the four demographic dimensions of species invasiveness. J. Ecol. 2016, 104, 1745–1758.
- Davies, K.W. Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 2011, 167, 481–491.
- Ström, L.; Jansson, R.; Nilsson, C. Invasibility of boreal wetland plant communities. J. Veg. Sci. 2014, 25, 1078–1089.
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol Env. 2009, 7, 142–149.
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676.
- Sanderson, L.A.; Antunes, P.M. The exotic invasive plant Vincetoxicum rossicum is a strong competitor even outside its current realized climatic temperature range. Neobiota 2016, 16, 1–15.
- Syamsuardi, N.Y.; Yulianti, W.; Usman, S. Floristic analysis of alien invasive plant species at some conservation areas in tropical forest of West Sumatra. Der. Pharm. Lett. 2016, 8, 237–245.
- Fine, P.V.A. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002, 18, 687–705.
- Denslow, J.S.; Dewalt, S.J. Exotic plant invasions in tropical forests: Patterns and hypotheses. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Wiley-Blackwell: Oxford, UK, 2008.
- Miguel, J.M.D.; Martín-Forés, I.; Acosta-Gallo, B.; Pozo, A.D.; Ovalle, C.; Sánchez-Jardón, L.; Castro, L.; Casado, M.A. Non-random co-occurrence of native and exotic plant species in Mediterranean grasslands. Acta Oecologica 2016, 77, 18–26.
- Ashbacher, A.C.; Cleland, E.E. Native and exotic plant species show differential growth but similar functional trait responses to experimental rainfall. Ecosphere 2016, 6, 1–14.
- Chen, B.M.; Li, S.; Liao, H.X.; Peng, S.L. Do forest soil microbes have the potential to resist plant invasion? A case study in Dinghushan Biosphere Reserve (South China). Acta Oecologica 2017, 81, 1–9.
- Mavimbela, L.Z.; Sieben, E.J.J.; Procheş, Ş. Invasive alien plant species, fragmentation and scale effects on urban forest community composition in Durban, South Africa. N. Z. J. For. Sci. 2018, 48, 1–14.
