Psoriasis is a chronic inflammatory autoimmune disorder that moderately affects social and interpersonal relationships. It is rapidly built up by skin surface cells which quickly form itchy and painful red patches. An everlasting cure for psoriasis is not available; nevertheless, its effect can be decreased by quitting smoking, moisturizing, and stress management.
- colloidal drug delivery system
- dendrimers
- liposome
- microemulsion
- nano-structured lipid carrier
- psoriasis
- solid lipid nanoparticles
1. Introduction
Psoriasis is a chronic inflammatory autoimmune disorder that moderately affects social and interpersonal relationships. It is rapidly built up by skin surface cells which quickly form itchy and painful red patches. An everlasting cure for psoriasis is not available; nevertheless, its effect can be decreased by quitting smoking, moisturizing, and stress management. Psoriasis is mainly proliferated by irregular keratinocyte differentiation and epidermal hyper-proliferation and is directly linked with diabetes and cardiovascular diseases [1,2,3]. It is an autoimmune acute or chronic disorder mediated by T-cells. It progresses by triggering the host’s immune system, which can be caused by keratin mediating cells multiplying and appearing on the skin’s surface. Usually, the keratinocyte cycle of development and replacement takes approximately 35–40 days, after which they shed off. However, in psoriasis, the maturation cycle takes about one week, and cells, instead of shedding off, accumulate on the skin’s surface to produce red lesions [4]. Biologics have been shown to be an excellent alternative therapy for people with moderate–severe psoriasis [5]. In the pathophysiology of psoriasis, tumor necrosis factor-α plays a crucial role. TNF-α levels are higher in psoriatics than in normal individuals, and the improvement is linked to the psoriasis area and severity index score. Psoriasis is increasingly regarded as a chronic inflammatory systemic illness mediated by multiple inflammatory cytokines, such as TNF-α, rather than a simple skin condition. TNF-α is key cytokine in psoriasis immune response activation, and also has important impacts on keratinocyte proliferation and the control of endothelium proteins required for T-cell migration [6]. Anti-TNF-α and anti-TNF-α receptor drugs are now being used to treat psoriasis [7].
Conventional treatments for psoriasis have certain problems, such as poor drug penetration through the skin, low aqueous solubility, poor bioavailability, asymmetric and erratic pharmacokinetic profiles, hyper-pigmentation, first-pass metabolism, and a burning sensation on normal and diseased skin. Colloidal drug delivery systems overcome the pitfalls of conventional approaches for psoriasis therapeutics. They have improved patient safety parameters, compliance, and superior effectiveness, in addition to reduced toxicity. In 2014, Marepally and colleagues incorporated two therapeutic nucleic acids, i.e., anti-STAT3 siRNA (siSTAT3) and anti-TNF-α siRNA (siTNF-α), into lipid nanoparticles using cationic amphiphilic oleyl chain-based lipids to develop fusogenic nucleic acid lipid particles (F-NALP). Topical delivery of F-NALP can transmit siSTAT3 and siTNF-α into the dermis and decrease the expression of STAT3 and TNF-α mRNAs to play synergistic role in treatment of psoriatic-like plaques [8]. Bessar et al. , in 2016, developed methotrexate (MTX)-loaded gold nanoparticles functionalized with sodium 3-mercapto-1-propansulfonate (Au-3MPS@MTX conjugate). Compared to MTX alone, the conjugate was superiorly percutaneously absorbed following 24 h application on naked mouse skin, and therefore, could be explored for effective treatment of psoriasis [9]. Wan and co-researchers, in 2017, fabricated hybrid nanoparticles (FK506 NPs-NIC) composed of hyaluronic acid with cholesterol combined with nicotinamide for tacrolimus (FK506) and illustrated synergistic action on FK506 permeation through intact skin. The PASI score in a imiquimod-induced psoriasis model demonstrated that FK506 HA–Chol NP–NIC exerted an ameliorating effect on skin lesions superior to commercial FK506 ointment [10]. In 2018, Nemati et al. developed a non-toxic fusion peptide carrier, i.e., spherical nucleic acid gold nanoparticles (SNA-NCs) conjugate with siRNA, in order to enhance the penetration of siRNA into cells, and found that topical application of SNA-NCs siRNA improved psoriatic-like skin lesions via suppression of gene expression and T-cell production [11]. In 2018, a group of researchers investigated discoidal lipid nanoparticles of APTstat3 tagged with a 9-arginine cell-penetrating peptide (APTstat3-9R). APTstat3 is an inhibitor of signal transducer and activator of transcription-3 (STAT3). It was found that transcutaneous delivery of lipid nanoparticles accomplished proficient skin penetration and successfully reduced psoriatic skin inflammation without producing adverse systemic effects [12]. Ramalheiro et al. , in 2020, generated encapsulated rapamycin using phytantriol-based cubosome-like liquid crystalline nanoparticles for transdermal and controlled release delivery, for psoriasis treatment, which showed a sustained drug release profile till 14 days and displayed in-vitro antiproliferative action in natural killer cells [13]. Recently, Fereig and colleagues, in 2014, developed chitosan nanoparticles with an anti-proliferative molecule, i.e., tacrolimus, which acts via T-lymphocytic cell inhibition. They reported skin deposition of 82% of the tacrolimus, which was significantly greater in comparison to pure Tacrolimus ® ointment, which showed about 34.0% skin retention [14].
Jyothi et al. also described traditional treatments along with nano-carriers and herbals used in psoriasis [15]. The current article gives brief information about conventional treatments for psoriasis and updated comprehensive information on recent advancements in various types of nanotechnology based colloidal drug delivery systems for treatment of psoriasis. The current article encompasses the colloidal drug delivery systems in several categories—viz. , emulsion systems, i.e., multiple emulsion, microemulsion, and nano-emulsion; vesicular systems, i.e., liposomes, ethosomes, noisomes, and transferosomes; particulate systems, i.e., solid lipid nanoparticles, solid lipid microparticles, nano-structured lipid carriers, dendrimers, nanocrystals, polymeric nanoparticles, and gold nanoparticles; and micelle systems, i.e., polymeric micelles, reversed micelles, and mixed micelles. Moreover, the recent advancements of such colloidal drug delivery systems, i.e., emulsion systems, vesicular systems, and particulate systems, have also been summarized in tabular form. Recent studies on herbal colloidal drug delivery systems for psoriasis management have also been incorporated in the present review. For this purpose, an extensive search of the literature was conducted using PubMed, Google Scholar, and ScienceDirect databases. The keywords used in the search strategy were “psoriasis”, “liposome”, “ethosome”, “niosome”, “transferosome”, “multiple emulsions”, “microemulsion”, “nano-emulsion”, “solid lipid nanoparticles”, “solid lipid microparticles”, “nanostructured lipid carrier”, “dendrimers”, “nanocrystals”, “polymeric nanoparticle” and “gold nanoparticle” in various combinations. This should help students and research scientists to better understand future research and development in the field of colloidal drug delivery-based treatment for psoriasis.
2. Pathogenesis of Psoriasis
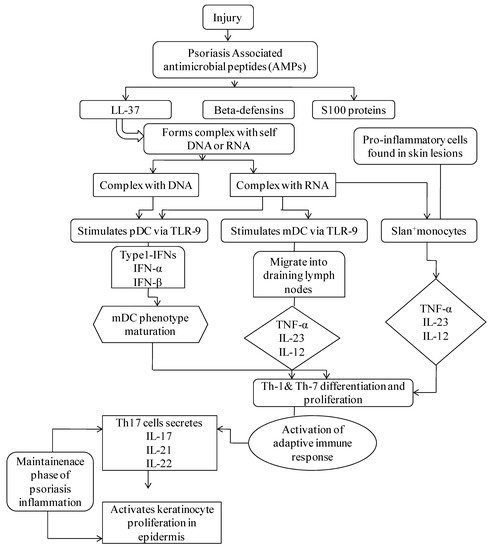
It has been commonly recognized that dendritic cells (DCs) are competent antigen-presenting cells that undertake a substantial role in several preliminary phases of the disease. The triggering of DCs in psoriasis, nevertheless, is not essentially precise. Several suggested pathways comprise the identification of antimicrobial peptides (AMPs) secreted through keratinocytes in reaction to trauma, which typically become over-expressed within psoriatic skin. LL37, S100 proteins, and β-defensins are major psoriasis-associated AMPs that are liberated through injured keratinocytes. LL-37 forms a complex with self DNA and RNA to produce LL-37-DNA and LL37-RNA complexes. The LL-37-DNA complex stimulates plasmacytoid DC (pDC) via toll-like receptor (TLR)-9, which discharges interferon-α (IFN-α) and IFN-β, leading to myeloid DC (mDC) phenotype maturation followed by Th1 and Th17 differentiation and function. Th17 cells secrete interleukins (IL)-17, IL-21, and IL-22, which activate keratinocyte proliferation in the epidermis. Complex LL-37-RNA triggers pDC via the TLR-7 pathway, which undergoes the above-described phases. Activation of mDC occurs through the TLR-8 mechanism, which migrates into draining lymph nodes that secrete tumor necrosis factor (TNF)-α, IL-23, and IL-12. These cytokines activate Th1 and Th17 differentiation and functions. Th17 cells discharge IL-17, IL-21, and IL-22, stimulating keratinocyte proliferation in the epidermis ( Figure 1 ) [16,17,18,19]. Genetic factors also play a primitive role in the pathogenesis of psoriasis. The genetic epidemiology mainly comprises factors such as twin studies, familial aggregation, heritability, susceptibility analysis, and pedigree analysis. The tendency of family aggregation in psoriasis is highly distinctive and varies according to the population. About 31.26% of the Chinese population has reported a family history of psoriasis—67% reported having primary relatives with the disease, and 47% having secondary relatives with it. Hence, genetic predisposition has been studied, and more than 80 susceptible loci have been identified to play roles in the pathogenesis of psoriasis. The related study of gene functioning is in full swing, and several modes of next generation sequencing technology are being used to develop more accurate and sensitive genetic markers which can be targeted by biologics to impart enhanced management of disease. The genetic findings have provided hints on the pathogenesis of the disease. Disease prevention and management have been focused on by developing advanced effective biologic treatments, in response. Some of the susceptible genes that can induce psoriasis are LCE cluster , AIM2, LRRC7, MTHFR, MGAT5, PSORS6, SLC12A8, PSORS5, PSORS 9, and LCE1-LCE6 .
3. Conventional Treatment Alternatives for Psoriasis
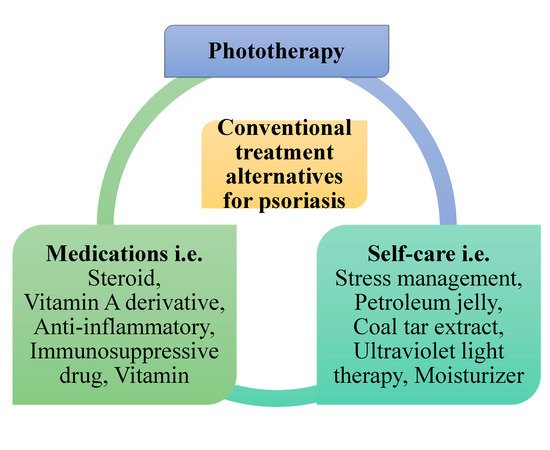
Conventional psoriasis treatments consist of phototherapy, self-care, and medications that aim to confiscate psoriasis scales and prevent skin cells from growing promptly ( Figure 2 ). Photodynamic therapy combines drugs with light therapy to destroy abnormal cells or close blood vessels. Self-care includes stress management; light therapy; ultraviolet light therapy; and applying petroleum jelly, coal tar extract, and moisturizer. Medications such as steroids work by reducing inflammation, slowing down the production of skin cells, and reducing itching. A vitamin A derivative unplugs blocked hair follicles and averts forming new blockages and decreases skin cell growth. Anti-inflammatory agents are mainly used to prevent or counteract inflammation in joints and tissues. Immunosuppressive drugs decrease the immune response. Vitamins helps with normal body functions, growth, and development. The current most effective topical treatment for psoriasis is a foam containing a steroid and a vitamin D derivative [35]. Nevertheless, all these conventional therapies have certain problems—i.e., poor drug penetration through the skin, hyper-pigmentation, and a burning sensation on normal and diseased skin in the case of topical therapy; and low aqueous solubility, poor bioavailability, and an asymmetric and erratic pharmacokinetic profile in the case of systemic therapy [1,2,3,15,26]. Systemic therapies based on traditional drugs such as cyclosporin and methotrexate, and biological therapies based on anti-tumor necrosis factor-alpha, anti-interleukin 17, and anti-interleukin 23 molecules, are used in severe cases with very good results [36,37].
4. Recent Advancements in Herbal Nano-Carriers for Psoriasis Treatment
The majority of herbal drugs are insoluble; hence, they have inadequate bioavailability and augmented systemic clearance, necessitating frequent administration or else high doses. Therefore, the development of a colloidal delivery system provides several benefits to phytoconstituents, such as improved permeability, reduced toxicity, augmented pharmacological strength, fortified stability, and sustained release. Consequently, the colloidal drug delivery systems of herbal ingredients have promise for improving the effects of, and conquering the troubles linked with, herbal medicines [115,116,117,118,119,120]. Meng et al. prepared a niosome gel loaded with celastrol and concluded that celastrol loaded into noisomes leads to a two-fold increase in drug penetration into the skin [119]. Pleguezuelos-Villa et al. developed a nano-emulsion loaded with magneferan to overcome the poor aqueous solubility and low bioavailability of magneferan, which is an anti-hyperproliferative and anti-inflammatory agent. It was concluded that the nano-emulsion improved bioavailability. Therefore, it could be a better treatment for inflammatory and skin disorders [121]. Divya et al. synthesized an acitretin and aloe-emodin-loaded chitin nanogel. They found deeper penetration of aloe-emodin into the tissue, thereby producing strong anti-inflammatory action to treat psoriasis [122]. Examples of several colloidal drug delivery systems for herbal constituents used for psoriasis management are represented in Table 8 .
| Herbal Constituent (Delivery System) | Excipients | Preparation Technique | Clinical Significance and Outcomes | Ref. |
|---|---|---|---|---|
| Celastrol isolated from Tripterygium regelii (Niosome) | Cholesterol, carbopol 934, span 20, span 60 | Thin film hydration | The developed nanoparticle has particle size of 147 nm and yield of up to 90% and increased water solubility and permeation of celastrol into skin which enhanced its anti-psoriasis activity in mice | [123] |
| Mangiferin isolated from leaves/bark of Mangifera indica (Nano-emulsion) | Lipoid® S75, hylouronic acid, Polysorbate 80 | Ultra-sonication | Nanoemulsions having mangiferin significantly reduce oedema ∼20-fold higher than empty nanoemulsions and reduce leucocyte infiltration and showed an anti-inflammatory activity | [124] |
| Acitretin and aloe-emodin Aloe-emodin isolated from plant of genus Aloe (Polymeric nanoparticle) | Chitin | Centrifugation | Revealed greater skin permeation and drug retention in deeper layer of skin with and improve compatibility | [125] |
| Tea tree oil isolated from leaves of Melaleuca alternifolia (Micro-emulsion) | Tween 80 | Emulsification | Showed superior drug solubilization and bioavailability for topical applications of anti-psoriatic active moieties and bio-actives | [126] |
| Curcumin (Nano-hydrogel) | Curcumin, choline-calix[4]arene amphiphile | Supra-molecular nano-hydrogel | Exhibited no significant toxicity and showed effective anti-psoriatic activity in an IMQ-induced psoriasis mouse via decreased pro-inflammation | [127] |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13111978
