Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Antimicrobial photodynamic therapy (aPDT) has become a fundamental tool in modern therapeutics to combat localized infections. Thanks to the expanding versatility of photosensitizers and the many possible combinations with other antimicrobial treatments, aPDT is evolving from a conceptually simple methodology towards a much more sophisticated, integrated, and innovative technology.
- antimicrobials
- combinatory strategies
- ROS
- photodynamic therapy
- photosensitizers
- multidrug resistance
- nanoparticles
1. Introduction
Antimicrobial resistance (AMR) occurring in bacteria, viruses, fungi, and parasites has been declared by the World Health Organization (WHO) as one of the top 10 global public health and development threats facing humanity. The misuse and overuse of antimicrobials make almost all disease-causing microbes resistant to drugs commonly used to treat them [1]. Multidrug resistance (MDR) to critical classes of antibiotics has gradually increased in nosocomial pathogens, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (which are gathered in the so-called ESKAPE group) [2]. Currently, in Europe, AMR is estimated to be responsible for 33,000 deaths every year and the annual economic toll, covering extra healthcare costs and productivity losses, amounts to at least EUR 1.5 billion [3][4]. Unless adequately tackled, 10 million people a year will die from drug-resistant infections by 2050, according to the predictions of the government-commissioned O’Neill report [5].
With the decline in the discovery of new antimicrobials since 1970s, the mainstream approach for the development of new drugs to combat emerging and re-emerging resistant pathogens has focused on the modification of existing compounds. However, one key recommendation encourages stimulation of early stage drug discovery [6]. Among emerging antimicrobial therapeutic alternatives, light-based approaches show particular promise [7]. Phototherapy was already a common practice in ancient Greece, Egypt, and India to treat skin diseases [8]. At the beginning of the 20th century, Oscar Raab first described the phototoxicity of the dye acridine red against Paramecium caudatum, and Tappenier and Jesionek reported the photodynamic effects of eosin suitable for treating diverse cutaneous diseases. Since then, photodynamic therapy (PDT) is established as the administration of a non-toxic photosensitizer (PS) followed by exposure to light irradiation at an appropriate wavelength focused on an area to treat [7].
Generally speaking, a given PS has the potential to produce reactive oxygen species (ROS) under specific conditions (Figure 1A). Typically, it possesses a stable electronic configuration called ground state level. Following irradiation and absorption of a photon, the PS is converted from a low (fundamental) energy level (1PS) to a “Frank Condon” short-lived, very reactive, excited singlet state 1PS* [9][10][11]. Subsequently, the PS can lose energy by emitting fluorescence or heat via internal conversion (IC), thereby returning to its initial ground state level; alternatively, it can be converted by a so-called inter-system crossing (ISC) to a longer-living excited triplet state 3PS*. From this state, two types of chemical reaction pathways can occur, known as Type I electron transfer and Type II energy transfer, which can take place simultaneously [12]. In the Type I reaction, the 3PS* captures an electron (e−) from a reducing molecule (R) in its vicinity, which induces an electron transfer producing the superoxide anion radical (O2●−) and, after the subsequent reduction, leads to the generation of more cytotoxic ROS including hydrogen peroxide (H2O2) and hydroxyl radical (HO●). In the Type II reaction, a direct energy transfer occurs from the 3PS* to the ground state molecular oxygen (3O2) that is then converted to singlet oxygen (1O2). The ROS thus produced encompass O2●−, H2O2, HO●, and 1O2, the last two being the most reactive and most cytotoxic species but also those with the shortest diffusion distance. One PS molecule can generate thousands of molecules of 1O2, depending notably on its 1O2 quantum yield, the surrounding environment, and the respective occurrence of Type I and Type II mechanisms [13][10][14].
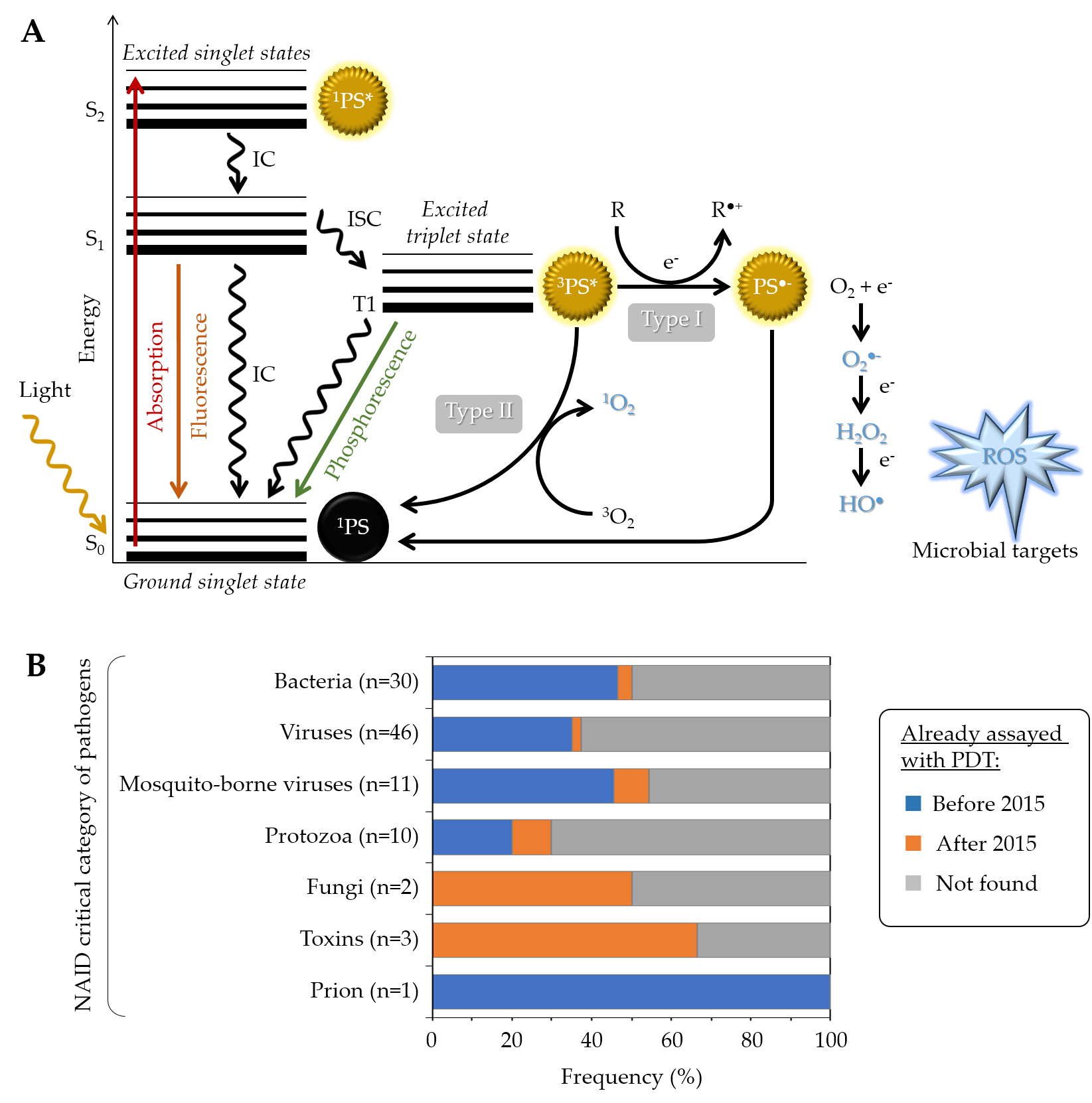
Figure 1. (A) Modified Jablonski diagram describing the photochemical and photophysical mechanisms leading to ROS production during PDT. (B) Overview of PDT already applied to the critical category of pathogens, as defined by the NIAID (https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens, accessed date: 1 September 2021). For each category, the chart specifies the number and the proportion (percent of pathogens already assayed in at least one PDT study either before or after 2015) [15].
In addition to the above-mentioned Type I and Type II mechanisms, Hamblin et al. recently proposed introduction of a Type III photochemical pathway, following which the radical anion PS•− and/or inorganic radicals formed in absence of oxygen could also lead to photoinactivation [16]. These authors indeed identified several circumstances in which oxygen-independent photoinactivation of bacteria using specific PSs can be obtained.
While anti-cancer PDT is a clinical reality for 25 years [17], PDT as an antimicrobial treatment was demonstrated for the first time against drug-resistant infections in the healthcare sector in the early 1990s, leading to a “photo-antimicrobial renaissance era” [7]. Since then, the number of studies dealing with antimicrobial PDT (aPDT) has dramatically increased, emphasizing the potential of this therapeutic approach to treat a broad spectrum of microorganisms, including bacteria [2][18][19][20][21][22][23], fungi [24][25][26][27][28][29][30][31], viruses [32][33][34][35][36][37][38], and parasites [39][40][41][42][43] (Figure 1B). Accordingly, multiple clinical trials have been conducted [15], mostly applied to the treatment of oral infections , but also skin infections including Acne [44][45][46], onychomycosis [47][48], osteomyelitis [49], or gastric ulcers [50]. At the current stage of development, aPDT cannot address systemic infections but it holds great promise for treating localized ones and to fight AMR.
Major MDR bacteria have been found susceptible to aPDT, independently of their drug-resistance profiles and resistance is rarely reported [51][52]. Given the multi-targeted nature of aPDT, the incidence of resistances is supposed to be very low [53]. However, micro-organisms may be able to respond to aPDT in different ways. For instance, light response adaptation can occur in some microorganisms, such as Escherichia coli upon exposure to blue light inducing the production of a biofilm polysaccharide colonic acid [54]. Moreover, some PSs are substrates of efflux systems that may be overproduced; specific inhibitors of the latter can be used to restore phototoxicity [55][56][57]. After sublethal aPDT, the biofilm-forming ability of bacteria can increase, making them less susceptible to the same treatment [58]. Each strategy should thus be carefully examined with regard to the ability of target microorganisms to adapt and escape treatments. The latter may be noticeably reduced when using at the same time multiple antimicrobial molecular partners and modalities. Thus, aPDT evolves towards increasingly sophisticated, integrated, and innovative strategies, leading to the concept of combinatory aPDT (c-aPDT) [15].
Following excellent earlier authoritative reviews [13][59][60][61], most recent developments since 2015 in the field of aPDT are highlighted herein, with a special emphasis on combinatory approaches [15]. It should be noticed that aPDT is sometimes referred to as photodynamic antimicrobial chemotherapy, light-based antimicrobial therapy, photo-controlled antimicrobial therapy, or antimicrobial photo-inactivation. All these synonyms were considered herein to present (i) a state of the art with recent developments in photo(nano)systems, (ii) the implementation of multicomponent nanotechnologies and recent molecular engineering, and (iii) the exploration of combinatory aPDT approaches towards possible future therapeutic innovations. This entry is adapted from 10.3390/pharmaceutics13121995.
2. State of the Art with Recent Photo(nano)System Developments
2.1. Single PSs
2.1.1. Organic PSs and Their Derivatives
Organic PSs used in aPDT have been well described in some recent reviews [62][63][64] (Figure 2A). Briefly, since the first use of eosin in 1904, various PSs were investigated, especially in the phenothiazinium group, which includes methylene blue (MB) and toluidine blue O (TBO). Thanks to an absorption spectrum in the red region of light, these PSs can be effective in tissues while being less toxic than other PSs. Their aPDT properties are mostly due to a high ROS production following Type I mechanism (Figure 1A). Structural derivatives have been also reported, including new MB and dimethyl-MB [65]. Another group gathers compounds featuring a macrocyclic structure composed of pyrroles, such as porphyrins and its precursor 5-aminolevulinic acid (5-ALA), phthalocyanines, and chlorins. Macrocyclic compounds are generally hydrophilic, positively charged, and they exhibit a good singlet oxygen quantum yield. Modifications of their chemical structure were intensively studied, especially for porphyrins and phthalocyanines [66][67][68][69][70][71]. The halogenated xanthenes gather PSs with a structure similar to that of fluorescein. Among them, eosin Y, erythrosine, and rose bengal (RB) were the most studied [72][73][74]. These compounds are anionic, which can limit their interaction with bacterial cells and their aPDT effect in spite of good singlet oxygen quantum yields. Natural compounds, including coumarins, furanocoumarins, benzofurans, anthraquinones, and flavin derivatives are often found in plants and other organisms. They are characterized by an absorption spectrum in white light or UVA. The most used are curcumin, riboflavin, and hypericin [7][63]. Nanostructures such as fullerenes are interesting PSs because of their ability to modulate Type I and Type II reactions (Figure 1A), depending on the near environment and the light source applied [75][76]. In this family, some quantum dots (QDs) can act as photoantimicrobials [63]. Other synthetic fluorescent dyes such as organoboron compounds (e.g., boron–dipyrromethene (BODIPY)), and cyanine dyes (e.g., indocyanine green (ICG)) are known for their high photostability, high extinction coefficients, and high fluorescence quantum yields [77].
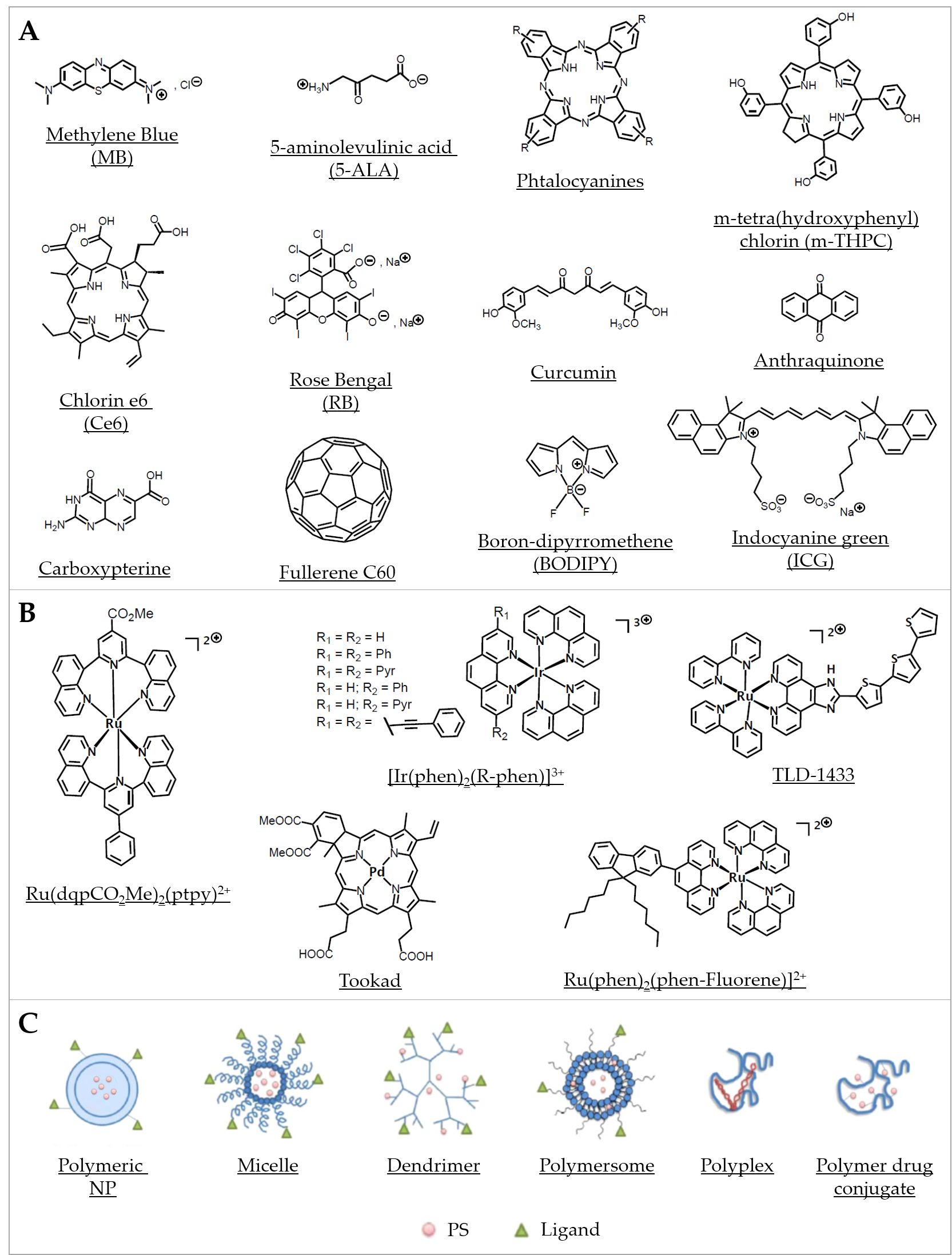
Figure 2. Representative compounds in various classes of PSs used in aPDT. (A) Examples of some organic PSs and their derivatives. (B) Examples of metallic-based PSs. (C) Different types of polymer-based PS carriers, which can be functionalized with ligands for specific target delivery (Adapted from [78], published by MDPI, 2020).
2.1.2. Coordination and Organometallic Complexes-Based PSs
Distinctly from metal nanoparticles (NPs; see Section 2.2.1 “Metal-based systems”), metal complexes, either coordination or organometallic complexes, are of increasing interest as PSs in PDT [79] (Figure 2B). They generally consist of a central metal core combined with ligands, involving coordinate covalent bonds (in coordination compounds) or at least one metal–carbon bond (in organometallic compounds). Compared with organic compounds, metal complexes have been notably much less considered and still remain largely underexploited regarding their potential use as new antibiotics [80]. Frei et al. recently reported on the antimicrobial activity of 906 metal-containing compounds. They showed that, considering antimicrobial activity against critical antibiotic-resistant pathogens, metal-bearing compounds displayed hit rates about 10 times higher than purely organic molecules [81].
Metal complexes display a panel of specific properties that make them promising PSs candidates. The variety of metal ions and ligands can be assembled in scaffolds featuring very diverse geometries [81][82]. Whereas most organic PSs are linear or planar molecules, metal complexes can exhibit much more complex—three-dimensional—geometries, which can improve interaction and molecular recognition with cellular targets, enlarge the spectrum of activity, and impact on biological fate [82][83][84]. Furthermore, the modulation of the design of metal complexes allows to fine tune their hydrophilic–lipophilic balance, solubility, photophysical properties, and eventual “dark toxicity” (i.e., the toxicity in the absence of specific irradiation). Metal complexes can display many excited-state electronic configurations associated with the central metal, the ligands, or involving both the metal and the ligand(s) in charge-transfer states (metal-to-ligand charge transfer or ligand-to-metal charge transfer). Although it is not always considered, the investigation of excited states of metal complexes (Figure 1A) is however of prime importance. Triplet states can be more easily accessed due to the enhanced spin-orbit coupling induced by the presence of the heavy metallic atom. Compared with natural PSs, metal complexes can act, besides ROS generation, via other mechanisms including redox activation, ligand exchange, and depletion of substrates involved in vital cellular processes [80][81][85].
Besides their first intended development as anticancer compounds, metal complexes have also been envisaged as potential “metallo-antibiotics”, benefiting from the knowledge accumulated about their chemical properties and biological behaviors [86]. Quite recently, several metal compounds were characterized for their activity in aPDT. For instance, platinum(II), molybdenum(II), ruthenium(II), cobalt(II), and iridium(III) were proposed as new classes of stable photo-activatable metal complexes capable of combating AMR [52][87][88][89][90][91][92]. In particular, many mononuclear and polynuclear Ru(II/III) complexes have been considered as potential antibiotics, antifungals, antiparasitics, or antivirals, which have been recently extensively reviewed [91]. It is worth noticing here that, within a series of 906 metal compounds, ruthenium was the most frequent element in active antimicrobial compounds that are nontoxic to eukaryotic cells, followed by silver, palladium, and iridium [81]. Ru(II) polypyridyl complexes were assayed in several aPDT studies. For instance, Ru(DIP)2(bdt) and Ru(dqpCO2Me)2(ptpy)2+ (DIP = 4,7-diphenyl-1,10-phenanthroline, bdt = 1,2-benzenedithiolate, dqpCO2Me = 4-methylcarboxy-2,6-di(quinolin-8-yl)pyridine) and ptpy = 4′-phenyl-2,2′:6′, 2″ -terpyridine) were tested with S. aureus and E. coli [93]. The complexes were found active against the Gram(+) strain and to a lesser extent against the Gram(−) strain, such difference in susceptibility being commonly reported in studies using other PSs [94] (Figure 2B). This observation was further detailed and rationalized by us when investigating a collection of 17 metal-bearing derivatives; two neutral Ru(II) complexes (Ru(phen)2Cl2 and Ru(phen-Fluorene)2Cl2) as well as a mono-cationic Ir(III) complex (Ir(phen-Fluorene)(ppy)2+; ppy = phenypyridyl ligand) were found almost inactive, whereas a dicationic Ru(II) (Ru(phen-Fluorene)(phen)22+) was found to be the most active against a panel of clinical bacterial strains [52]. More recently, Sun et al. described a Ru(II) complex bearing photolabile ligands; they showed its ability to safely photoinactivate intracellular MRSA while inducing only negligible resistance after bacterial exposure for up to 700 generations [95]. Although the precise mechanism(s) of action is not well-established in every case, Ru(II/III) compounds are also increasingly considered for their potential anti-parasitic activity for combating neglected tropical diseases such as malaria, Chagas’ disease, and leishmaniasis. Moreover, potent antiviral activities have been noted for the ruthenium complex BOLD-100, particularly against HIV and SARS-CoV-2 [96]; importantly, this compound appears to retain its activity on all mutant strains of the SARS-CoV-2 [91]. All combined, metal complexes—especially ruthenium-based compounds—can display antimicrobial activity via multiple, likely synergistic, mechanisms, involving notably their ability to produce ROS. Therefore, they are of major interest for a wide range of aPDT-related applications.
2.2. Multicomponent PSs and Nanoscale Implementation: Extension to Nanoedifices with PSs
The eventual limitations in the use of singular photoactive molecules as PSs for aPDT applications reside notably in the recurrent lack of solubility and stability in the target media (typically leading to aggregates and/or PS quenching), biocompatibility (“immune stealth”) and bioavailability, but also in the relative absence of selectivity for a prospected target (e.g., efficiency in the interactions with a defined target, stimuli-responsive or alternate triggers for controlled release). Thus, the widespread nanoscale implementation into the development progress of upgraded PSs has indubitably provided significant flexibility to first address these drawbacks, and implicitly contribute to the optimization of the aPDT activity via an extensive panel of mainly exclusive features specific to nano entities; the latter typically include an advantageous surface to volume ratio (with a high PS per mass content), access to unique chemical/physical/biological properties (e.g., optical properties with QDs or magnetic ones with superparamagnetic iron oxide NPs (SPIONs)), and almost inexhaustible synthetic options in the design of nanoplatforms [97][98][99].
Due to their paramount structural diversity and intrinsic variety of properties, the classification of the so-called “PS nanosystems” or “nano-PSs”—which partly include and overlap with “conjugated systems” and “combinatorial strategies”—remains rather challenging; however, we can usually identify the following criteria as the main pillars to rationalize and compare these aPDT nanomaterials [100][63][101]:
- (i)
-
Role(s) and nature of the nanocomponent(s) in the PS nanosystems: the two criteria typically considered for the discrimination of nano-PSs are the role and nature of the nano building block(s) involved. With regards to the role of the latter, we can conventionally discern on the one hand the “PS nanocarriers” (e.g., polymersomes or Au NPs) in which the nanomoiety acts as a delivery system for singular PS molecules (e.g., MB) while either complementing, facilitating, or enhancing the aPDT activity (depending on the nature and eventual intrinsic properties of the nanovector), and on the other hand, the “PS active” nanoagents with the nanocomponent endorsing the role of PS. Among the examples, some versatile nanotemplates may ultimately display a dual role, i.e., “active PS” and “PS conveyor” (e.g., ZnO NPs), while distinct nanomoieties might be simultaneously required for the design of utterly sophisticated hybrid nano-PSs (e.g., Au@AgNP@SiO2@PS) [102][103]. In addition to the chemical composition, the nature of the nano building block(s) will also be defined by the fundamental characteristics of nano-objects, such as size, shape, topology, and crystal structure, which will all ineluctably contribute to tailor the biological behavior of the nanomaterials and the interactions with the targets (e.g., with the membranes of the bacteria) [104]. Moreover, for the same nanocore, the nature and role of the eventual surfactant(s) involved (e.g., silica coating or poly(ethylene glycol) (PEG) coating for metal NPs) can drastically alter the overall behaviors of the nanosystems.
- (ii)
-
Type of interactions between the nano entity and the PS, and localization of the PS in the nanosystems: other criteria of relevance when describing nano-PSs—specifically PS nanocarriers—reside in the nature of the interactions between the nanocomponent and the PS molecules involved, but also the location of the PSs. Thus, we can distinguish the common cases of PS molecules “embedded” within a nanovector either by physisorption or functionalized (chemisorption), and alternately the nanoplatforms with surfaces decorated with PSs, again, either by physical or chemical adsorption. The differences between the two types of interactions and distinct localizations of the PSs implicitly imply distinctive chemical engineering and related requirements, and may potentially impact the resulting stability of the nanoedifices, but also the aPDT activity. For instance, in the case of PS molecules located inside the edifice and not released, the selected “nanomatrix” should adequately permit the photoactivation process of the internalized PSs, be sufficiently porous/permeable to both triplet and singlet oxygen and eventual ROS generated by the photosensitizers (i.e., efficient internal diffusion of molecular oxygen to react with the PSs then external diffusion of 1O2 to the targets) while also presenting inertness to the latter to not compromise or quench the aPDT activity. Meanwhile, with surfaces of nano-objects decorated with PSs, the PSs may then contribute to some extent as an interface with the biological medium or the target.
- (iii)
-
Biological impacts of the PS nanosystems: in addition to the biocompatibility and aPDT efficiency (including the critical concentrations just as the half-maximal effective concentration EC50, minimum inhibitory concentration MIC, or 50% growth inhibition concentration GIC50), the eventual biodegradability, elimination process, or ecotoxicity of the aPDT nanomaterials can markedly vary from one system to another (based on factors such as composition and size/shape), but are rather difficult to evaluate or compare; ergo, these factors are not systematically addressed in the reports.
- (iv)
-
Relative sustainability of the nano-PSs for aPDT applications: the reproducibility, eco-friendliness, and cost-effectiveness parameters of the synthetic protocols and production of aPDT nanomaterials, as well as the ease of storage and use, and the stability over time are also ultimately to be evaluated for any system aiming to be viable and reasonably applied; however, similar to (iii), these parameters are complex and so scarcely investigated.
Thus, in the overview presentation of the different PS nanosystems hereafter, the chemical features (i) and (ii) have been conventionally defined as the main criteria for the classification. Alternatively, the nature of the aPDT applications has also been used as the main criterion for classification in some references [105]. Another approach consists of systemizing all the nanosystems dedicated to a given PS (e.g., curcumin) [106].
Within the extensive collection of aPDT nanomaterials reported to date, the majority belongs to the category labeled as “PS nanocarriers” with the nanocomponent acting as a delivery system for PS molecules; however, increasing examples involving PS-active nano building blocks have emerged as well. Overall, this multipurpose role of the nanomoiety may include avoiding aggregation (e.g., dimerization, trimerization) and correlated PS quenching, enhancing “solubility” (i.e., dispersibility), stability and bioavailability, allowing “biological stealth”, on-demand release and target specificity, and ultimately triggering eventual synergistic aPDT activity with the complementary or ameliorative intrinsic properties of the nanocomponent; although irrevocably confirmed in many nano-PSs, the mechanisms involved in the synergy may differ from one system to another, and often remain partly or integrally unresolved due to the complexity of these tacit multiparameter contextures [97]. It is noteworthy that most systems comprise “classic”/”traditional” organic PSs (natural or synthetic, e.g., curcumin, MB) with fewer examples involving metallated PS molecules such as the recent review from Jain et al. dedicated to ruthenium-based photoactive metalloantibiotics [92]. Among aPDT nanomaterials, we can thus identify various families of nanoplatforms based on the nature of the nanocore, starting here with the inorganic vectors followed by the organic templates; as an indication, in the common cases of “multi-component nano-PSs”, the classification has been defined hereafter according to the main/prominent nano building block involved in the composition, i.e., metal-based systems, silicon-based systems, carbon-based systems, lipid-based systems, and polymer-based systems. Regarding the following presentation of aPDT nanosystems, it is important to specify that it is not exhaustive, but instead provides a panorama of the main categories of nanosystems—either colloids or surfaces [100][107]—and their related specificities, with an emphasis on recent developments.
2.2.1. Metal-Based Systems
Metal-based nanostructures have been extensively investigated—both as “PS cargo” and PS active entities—through the exploration of the richness and diversity of the respective subcategories related to this class of compounds, as detailed below. Each of the below-mentioned inorganic classes presents distinct specificities of relevance for aPDT applications, with a choice to be defined on a case-by-case basis according to the target, the nature of the PSs involved (with possible preferential affinity), or the anticipated complemental or synergistic role of the selected nano entity (based upon its chemical, physical, and/or biological features, with the eventual PS molecules located either at the surface of the NPs or within, when present). It is important to notice that the nanocores from each subcategory can be either further implemented, with silica or polymer coating for example, or combined (multicomponent nano-PSs) in order to adequately optimize the efficiency of the systems [102]; however, the outcomes of these hybridity processes are complex to anticipate with systematic rationality, with either enhancement or quenching of the properties observed depending on the composition of the combinations.
Metal NPs
Metal NPs—mainly gold, but also silver—maybe considered among the “gold standards” in nanomaterials through the history and expansion of nanosciences in terms of dedicated publications and vastness of related possible applications [108]. As already well documented for Au and Ag NPs, the reasons are numerous and reside notably in their relatively easy accessibility with low-cost and highly reproducible (large scale) biogenic and chemical synthetic routes, in addition to the flexibility to finely tailor the properties via a refined size and shape control (with narrow size distribution and diverse shapes), and the facility for functionalization with various types of molecules. Moreover, gold NPs display biocompatibility, low toxicity, and immunogenicity, almost chemical inertness (distinctly from their inherent catalytic properties), while silver nanomaterials present intrinsic antimicrobial activity against a broad spectrum of microorganisms and related MDR infections (e.g., towards Gram(−) and Gram(+) mature biofilms of MRSA), and disruption of biofilm formations while being safe for mammalian cells [109][110][111].Ultimately, both Au and Ag NPs share nano features specific to noble metal systems, i.e., localized surface plasmon resonance (LSPR, arising from their resonant oscillation of their free electrons upon light exposure) and resonance energy transfer (RET), with subsequent optical and photothermal properties of enhancing appositeness in aPDT applications and PDI efficacy (e.g., ROS production) [112][113][114]. For the most part, Au and Ag NPs of various shapes (e.g., spheres, rods, cubes) are combined with organic PS molecules such as RB and MB [113][114][115][116][117][118][119][120][121], but also with metallated PSs such as ruthenium complexes, metallophthalocyanines, and metalloporphyrins [92][122][123]; the corresponding PS nanovehicles can also be labeled as “conjugates” but they strictly differ from the “mixtures” involving metal NPs and PS molecules [124]. Other noble metal NPs, viz., platinum, have also been employed in aPDT applications due to their multitarget action to inactivate microbes, although to a lower degree up to now owing to synthetic limitations [100][125]. Alternately, redox-active copper NPs are typically less costly and easier to access and present unique features among which the faculty to generate oxidative stress to various microbes through the genesis of ROS [126], such as in the recently developed copper–cysteamine (Cu–Cy) nano-PS that can be activated either by UV, X-ray, microwave, or ultrasound, to produce ROS against cancer cells and bacteria [127][128]. More unwontedly, approaches to treat subcutaneous abscesses lead to the use of acetylcholine (Ach) ruthenium composite NPs (Ach@RuNPs) as an effective appealing PDT/PTT dual-modal phototherapeutic killing agent of pathogenic bacteria, with Ach playing a role in targeting the bacteria and promoting the entry into the bacterial cells [92]. While belonging to the same category, each metal displays particular specificities; consequently, with the objective of optimization, nano-PSs resulting from alloys or multimetallic NPs have been further designed such as the Au@AgNP@SiO2@PS and AA@Ru@HA-MoS2 (AA: ascorbic acid, HA: hyaluronic acid) nanocomposites [102][103].
Metal Oxides
Similar to gold and silver, metal oxides such as iron oxide, titanium oxide and zinc oxide have been cornerstone contributors in the global evolution of applied nanomaterials (particularly in medicine), due notably to intrinsic magnetic and optical nanoscale features, with the latter typically available at “room temperature” and commonly finely tunable via shape, size, and crystal structure parameters [129]. Indeed, specific single-domain superparamagnetic iron oxide NPs (SPIONs)—either magnetite (Fe3O4) or maghemite (γ-Fe2O3) of various shapes and sizes, including ferrite or doped derivatives—exhibit an outstanding magnetization behavior with no remanent or coercive responses upon exposure to a magnetic field. As a result, such magnetic NPs have legitimately generated interest and use as magnetic resonance imaging (MRI) and magnetic particle imaging (MPI) agents, as well as magnetic fluid hyperthermia (MFH), magnetic cell separators [130], or drug delivery conveyers with the possibility to guide the NPs to the targeted area via external magnetic fields [131]. More recently, iron oxide nano-objects proved to be also of pertinence for aPDT applications not only as a magnetic “nanocargo” for various organic and inorganic PSs such as curcumin, MB, ICG, BODIPYs, porphyrins, metallophtalocyanines, or ruthenium derivatives among others [100][92][132][133][134][135][136][137][138][139][140], but also with peroxidase-like activity to enhance the cleavage of biological macromolecules for biofilm elimination [141]. Extension in the design of more elaborate multicomponent architectures involving hybrid iron oxide nanocore led inter alia to Ag/Fe3O4, Ag/CuFe2O4, CoFe2O4, and Fe3O4/MnO2 NPs conjugated with different PSs [100][102][132][135][136][142][143][144][145].
Other oxides have also drawn heavy attention, in particular zinc oxide and titanium oxide, as the photophysical properties of these wide bandgap semiconducting nanomaterials efficiently translate into a multi-level antimicrobial activity including PS vessel and/or PS active agent (with possible coupled aPDT response), and/or membrane disruptor [146][147][148][149]. Thus, ZnO and TiO2 nanoplatforms possess the ability to alter microbes’ integrity—through alternate mechanisms involving ROS and/or metallic ions—in the dark or via photoactivation [150]. The latter is customarily triggered by UV or X-ray irradiation, with the eventual possibility to adequately shift to other wavelengths such as visible light irradiation in virtue of diverse modification methods of the oxides encompassing notably: doping or surface alteration (e.g., F-doped ZnO, coatings or oxygen deficiency), coupling with other bandgap semiconductors (e.g., ZnO/TiO2) or sensitizing dyes, and composites [106][141][149][151][152][153][154][155][156]. Furthermore, beside the size and shape of the NPs, the crystallographic phase appears to be a tuning parameter of particular importance for the antimicrobial effects of some oxides, especially for TiO2 with the distinction between the anatase, rutile, and brookite structures [157][158][159][160]. Although reported to a lesser extent, the list of alternate oxides exhibiting potential in aPDT applications comprises CuO/Cu2O, MnO2, and rare earth oxides to mention just a few [126][161][162][163][164][165].
QDs and Metal Chalcogenide Nanomaterials
Aside from zinc/titanium oxides, distinct semiconductors such as QDs and metal chalcogenide (e.g., metal sulfide) nanomaterials (involving elements from different groups in the periodic table) proved to be efficient disruptors against various multi-drug-resistant microorganisms. Due to their smaller size of a few nanometers (ca. up to 10 nm), QDs differ from other nano-objects with physical and optoelectronic properties governed by the rules of quantum mechanics, high chemical stability and resistance to photobleaching, and near-infrared (NIR) emission (typically above 700 nm) notably allowing for deep-tissue imaging [166]. Appositely comparable, metal chalcogenide likewise reveals unorthodox physio and physicochemical properties, accordingly garnering a legit interest for antimicrobial applications [167]. Consequently, not only can these nano building blocks carry PS molecules and alter the integrity of microbial walls/membranes or gene expression, but they may as well act as PSs; when coupled with other PSs, synergistic interactions in the QD-PS edifices might occur resulting from mechanisms such as Förster resonance energy transfer (FRET, non-radiative energy transfer from QD donors to PS acceptors) to generate free radicals and ROS. Among examples of such QDs and metal chalcogenide aPDT systems can be cited CdTe QDs and related CdTe-PS conjugates, CdSe/ZnS QDs combined with PSs, InP and InP-PS, Mn-doped ZnS, MoS2, but also CuS and CdS nanocrystals, with ultimately hybrid systems involving for instance CoZnO/MoS2 or AgBiS2–TiO2 composites [108][168][169][170][171][172][173][174][175][176][177][178][179][180][181].
Metal–Organic Framework (MOF) Nanoscaffolds, Upconversion Nanomaterials and Other Metal Ion Nanostructures
Although less investigated than the above-mentioned alternatives, other original metal-based nanostructures identified as MOF nanostructures, upconversion nanoplatforms, and alternate metal ion nanomaterials tend to further consolidate their promising potential for aPDT applications. Considering their towering surface area and porous ordered structure with substantial loading capacity (e.g., adsorption of O2 and ensuing photocatalytic production of 1O2 via a heterogeneous process), stable versions of colloidal nano-MOFs—or less common covalent organic frameworks (COFs), i.e., reticulation variants typically defined by non-metal “nodes” instead of metal ones—have emerged as efficient heterogeneous photosensitizers—with frameworks acting as PSs or entrapping PSs—towards antimicrobial applications (e.g., enhanced penetration for bacterial biofilms eradication), with porphyrin-based or porphyrin-containing MOFs and COFs, or Cu-based MOFs embedded with CuS NPs for rapid NIR sterilization among recently reported solutions [182][183][184][185][186][187][188][189][190].
Moreover, upconversion NPs (UCNPs) generally involve actinide- or lanthanide-doped transition metals and refer to the nonlinear process of photon upconversion, viz., a sequential absorption of two or more photons resulting in an anti-Stokes type emission (i.e., emission of light at a shorter wavelength than the excitation wavelength); when translated into biomedical context, UCNPs can be typically activated by NIR light—characterized by deeper tissue penetration and reduced autofluorescence, phototoxicity, and photodamage when compared with UV or blue light—and produce high energy photons for optical imaging or more recently aPDT when combined with PSs [191][192][193][194]. Intrinsically limited by low upconversion quantum yield, the current focus consists of developing hybrid UCNPs to improve aPDT efficiency; thus, auspicious progress has been achieved with examples such as {UCNPs (NaYF4:Mn/Yb/Er)/MB/CuS-chitosan)} multicomponent nanostructured system revealing a superior antibacterial activity with the UCNPs enhancing the energy transfer to MB, the CuS triggering synergistic PDT/PTT effects, and chitosan assuring stability and biocompatibility [141]. Other examples also include silica coating β-NaYF4:Yb, Er@NaYF4 UCNPs loaded with MB as PS and lysozyme as a natural protein-inducing bacterial autolysis, Fe3O4@NaGdF4:Yb:Er combined with the photo/sonosensitizer hematoporphyrin monomethyl ether (HMME), UCNPs@TiO2, N-octyl chitosan (OC) coated UCNP loaded with the photosensitizer zinc phthalocyanine (OC-UCNP-ZnPc), or the UCNPs-CPZ-PVP system (CPZ: β-carboxy-phthalocyanine zinc, PVP: polyvinylpyrrolidone) to name but a few [191][195][196][197][198].
Alternatively, more disparate metal-ion aPDT systems have been reported such as PS encapsulated dual-functional metallocatanionic vesicles against drug-resistant bacteria involving copper-based cationic metallosurfactant, or self-assembled porphyrin nanoparticle PSs ZnTPyP@NO using zinc meso-tetra(4-pyridyl)porphyrin (ZnTPyP) and nitric oxide (NO) [199][200][201].
2.2.2. Silicon-Based NPs
This category will be divided hereafter into two main subclasses—porous silicon (pSi) and (mesoporous) silica (SiO2)—which differ in the oxidation state of the silicon and display distinctive properties, more specifically different quenching behavior and photodynamic activity (singlet oxygen quantum yield under irradiation).
Porous silicon NPs (pSi NPs) are among the most promising types of inorganic nanocarriers for biomedical applications and have been intensively investigated since the first publication by Sailor et al. in 2009 regarding their application for in vivo treatment of ovarian cancer. Composed of pure silicon, pSi NPs indeed exhibit relevant features encompassing not only pores with large capacity for drug loading combined with specific surface area allowing for implemental functionalization, but also degradability in an aqueous environment, and biocompatibility [202]. Moreover, porous silicon particles are known to be photodynamically active with related inherent antimicrobial properties by generation of ROS under irradiation with light of a specific wavelength [203][204]. Because of the low quantum yield of singlet oxygen production from porous silicon itself, particles can be grafted with additional PSs, such as porphyrins, to enhance the yield of singlet oxygen generation and thereby antimicrobial properties for PDT applications [204][205]. Consequently, several silicon-based systems have been reported in recent years, mainly for PDT applications [206][207]. Furthermore, pSi NPs display intrinsic fluorescence, which can be applied for imaging and real-time diagnostics regardless of any surface functionalization [205][208].
As previously mentioned, pSi has a low singlet oxygen quantum yield due to quenching, which makes silica particles in comparison a more suitable substitutional system combining similar biocompatibility with improved optical properties. On the other hand, one of the pivotal advantages to use silica (SiO2) conjugates with PSs is to achieve a better “solubilization”—or more accurately, dispersibility—of hydrophobic dyes and a better photostabilization, thus limiting the self-photobleaching of PSs. Further advantages to name as the most important features of silica are high biocompatibility, antimicrobial properties, and high surface area for mesoporous silica that can be synthesized easily from commercially available precursors [100][209][210]. Furthermore, SiO2 exhibits an effective PS-grafting capacity [211]; the latter can be accomplished via adsorption, covalent bonding, binding to the hydroxyl groups from silica surface, and entrapment during formation in silica particles or matrix [100]. Recently, Dube et al. reported about the photo-physicochemical behavior of silica NPs with (3-aminopropyl)triethoxysilane (APTES), and subsequently PS-modified surfaces for aPDT [135]. In addition, silica coatings have also been reported to prevent the degradation of nanocarriers (magnetite) and prolong the stability and functionality of PS systems [212]. Interestingly, coupling PSs to silica or Merrifield resin leads to distinct advantages; indeed, immobilized Ce6 notably displays significantly higher aPDT efficiency in comparison with the free form, which is probably due to an enhancement of the adhesion of PSs to bacterial cells resulting in a stronger cell wall disorganization [213][214]. Unsurprisingly, many approaches with encapsulated PSs in silica NPs for potential aPDT applications have been reported in recent years [214][215][216][217].
Distinctly, combinatory approaches involving other nanocomponents, such as silica-containing core-shell particles or silica-coated inorganic NPs, have emerged to further implement the properties of silica with the specific features (e.g., magnetic, photoactive, or antimicrobial properties) from other nanomaterials of relevance [103]. Thus, since the surface of silica can be easily grafted with PSs and is highly biocompatible, the surface modification of silica particles using metallic NPs (e.g., Ag NPs) to enhance the antibacterial photodynamic activity, has been developed for improved aPDT [218] while combinations with carbon quantum dots have been assessed for imaging-guided aPDT [219]. Another example refers to sufficient aPDT/PDI systems, more precisely to mesoporous silica-coated NaYF4:Yb:Er NPs with the PSs (silicon 2,9,16,23-tetra-tert-butyl-29H,31H-phthalocyanine dihydroxide) loaded in the silica shell to enhance bacterial targeting of E. coli and S. aureus [220].
In addition to silica-based NPs or silica-containing core-shell particles, lesser-known silica nanofibers also proved to be suitable substrates for potential aPDT, PDT, and PDI applications. As an illustration, Mapukata et al. [221] recently reported silver NP-modified silica nanofibers with embedded zinc phthalocyanine as PS for aPDT applications. The nanofiber-based substrates offer the advantage of fast removal after application, which can allow limiting any dark toxicity [221][222][223].
Silica NPs and fibrous or dendritic fibrous nanosilica have also been reported for the formation of nanocomposites to create antimicrobial photodynamically active surfaces for aPDT or PDI; to create such surfaces, silica NPs can be embedded into polymeric matrices for enhanced biocompatibility and complementary surface properties from the selected polymer [224].
Additionally, silica substrates and nanoconjugates offer a suitable platform for combinatory approaches since they can be easily modified. For example, Zhao et al. described polyelectrolyte-coated silica NPs modified with Ce6 [225]. These complexes could be extracted, by bacteria, from silica NPs to form stable binding on the bacterial surface, changing the aggregation state of Ce6 and leading to both the recovery of PS fluorescence and 1O2 generation. Such bacteria-responsive multifunctional nanomaterials allowed for simultaneous sensing and treating of MRSA. Another approach is illustrated by the photo-induced antibacterial activity of amino- and mannose-decorated silica NPs loaded with MB against E. coli and P. aeruginosa strains [226]. The modification of silica substrates with mannose led to an increased targeting of P. aeruginosa and reduced dark toxicity of the systems.
2.2.3. Carbon-Based Nanomaterials
Akin to silicon-based nano-objects, the notable diversity of allotropic customizable carbon-based nanostructures—either conveyers for traditional PS molecules or intrinsically PS active—legitimizes their distinct consideration in the actual classification of nano-PSs [227][228][229], with the following subcategories.
Fullerenes, Carbon Nanotubes (CNTs), and Nanodiamonds
In the evolution of carbon-based nano-objects, fullerenes and carbon nanotubes derivatives may be chronologically introduced as the “first generation”. Discovered in the mid-1980s, the proper Cn (n = 60–100) spheroidal “soccer ball” π-conjugated structures of the fullerenes yield tremendous chemical modularity and electrochemical and physical properties, including photostability, the propensity to act as a PS via Type I or Type II pathways (Figure 1A) with high ROS quantum yield, and oxygen-independent photo-killing by electron transfer. Despite their intrinsic hydrophobicity typically requiring surface functionalization for biocompatibility and related dispersibility, they have proven even nowadays their effectiveness as broad-spectrum photodynamic antimicrobial agents, with photoactive antimicrobial coating based on a PEDOT-fullerene C60 polymeric dyad (PEDOT: poly(3,4-ethylenedioxythiophene)), BODIPY-fullerene C60, diketopyrrolopyrrole–fullerene C60, and cationic fullerene derivatives among recent examples [63][76][141][230][231][232][233][234][235][236]. Mainly developed a few years later, carbon nanotubes (CNTs)—either single wall CNTs (SWCNTs) describable simply as a single-layer sheet of a hexagonal arrangement of hybridized carbon atoms (graphene) rolled up into a hollow cylindrical nanostructure, or multiwall CNTs (MWCNTs) consisting of nested SWCNTs—unveil both independent capacities to produce ROS upon irradiation and high surface area for decoration with PS molecules [141]. Neoteric specimen of PS-CNTs encompass toluidine blue, polypyrrole, malachite green, MB, RB, and porphyrins [141][237][238][239][240][241][242]. Although purportedly older since it was discovered in the 1960s, diamond NPs or nanodiamonds seemingly remain the lesser known carbon-based nanomaterials to date; nevertheless, the latter dispose of legit aPDT arguments with their fluorescence, photostability, proclivity for conjugation with diverse PSs such as porphyrins or metallated phthalocyanines and silver NPs, but also inherent antibacterial activity [105][243][244][245][246][247].
Carbon QDs (CQDs)
As the next momentum in the blossoming of “nano-carbon” era, the carbon QDs were discovered in the early 2000s [248][249]. The physical and chemical properties of these fluorescent particles, commonly quasi-spherical with less than 10 nm in diameter, can be finely tuned upon size/shape variations or doping with heteroatoms (e.g., B, N, O, P, S) [248][250]. By virtue of their biocompatibility and dispersibility, photostability, low toxicity and related eco-friendliness, good quantum yield and conductivity, CQDs have been investigated for various applications, and more recently as antimicrobial agents; withal, their environment-friendly features combined with low cost and rather ecological biogenic or synthetic routes (from natural or synthetic precursors) place them advantageously as a viable scalable photocatalytic disinfection material compared with alternate nano-PSs. Late cases involve doped or hybrid CQDs or more conventional conjugates of CQDs with PSs [106][251][252][253][254][255][256].
Graphene, Graphene QDs (GQDs), and Graphene Oxide (GO) Nanostructures
In a similar timescale to CQDs is the quantum leap discovery and blooming of graphene and graphene oxide materials. Graphene can be defined as a 2D allotrope of carbon, more accurately a monolayer of atoms with a hexagonal lattice structure (or single-layered graphite) and identifiable as the “building block” for the discrete fullerenes, 1D carbon nanotubes, and 3D graphite. Despite its stunning mechanical/electronic properties and chemical inertness, the limitations of graphene, such as zero bandgap and low absorptivity, lead to the ulterior conversion of the 2D graphene into “0D” GQDs [257][258]. Due to quantum confinement and edge effects, GQDs exhibit different chemical and physical properties when compared with other carbon-based materials, as well as a non-zero bandgap, good dispersibility, and propensity for functionalization and doping. Structurally, GQDs differ from CQDs because they comprise graphene nanosheets with a plane size less than 100 nm [257][259]. Likewise, graphene oxide (GO) is the oxidized form of graphene i.e., a single atomic sheet of graphite with various oxygen-containing moieties either on the basal plane or at the edges. Meanwhile, reduced graphene oxide (rGO) can be summarily described as an “intermediate” structure between graphene and GO, with variable and higher C/O elemental ratios compared with GO, but remaining residual oxygen and structural defects with reference to the pristine graphene structure. Although GO was reported a couple of centuries ago, GO and rGO nanomaterials have mainly emerged for various applications after the discovery of graphene since GO is a precursor to prepare graphene, and both present distinctive physical and chemical properties that differ from graphene. As a result, countless and rising fast illustrations of graphene derivatives for antibacterial applications are regularly reported [106][260][261][262][263][264][265].
4.2.4. Lipid-Based Systems
Due to their amphiphilic nature (typically hydrophilic “head” and hydrophobic “tail”), some lipids—natural and synthetic—have been extensively studied to develop efficient biocompatible delivery systems—initially for drugs and DNA/RNA, but also for aPDT PSs—with synthetic flexibility and structural diversity. Among the prevalent examples, we can distinguish the micelles (lipid monolayers with polar units at the surface and hydrophobic core) and the liposomes (one or more concentric lipid bilayer with a hydrophilic surface and an internal aqueous compartment). Although sharing similar chemical constituents, the micelles and liposomes present significant differences to be taken into consideration depending on the intended application (nature of the target) and the nature of the PSs. Indeed, the micelles are typically smaller than liposomes (with a diameter starting from a few nanometers for the micelles, and ca. 20 nm for the liposomes), with distinct stability and permeability in biological medium and uptake pathways for the PSs into bacteria. With reference to the nature of the transported PSs, the liposomes display the additional flexibility to carry both hydrophilic PSs (in the core compartment or between the bilayers) or/and hydrophobic PSs (within the lipid bilayer), while the micelles are usually easier and cheaper to prepare [63][141]. As often critical to address for biomedical applications, the surface charge of these nano-objects can be tailored to further optimize the interactions with the bacteria, with cationic modification of liposomes identified as a promising aPDT efficiency “amplifier” [63][141][266]. In addition to recent examples such as the hypericin loaded liposomes against Gram(+) bacteria [267][268][269][270][271], another emerging and promising alternative includes the development of modified liposome-like derivatives labeled either as “ethosomes”, “transfersomes”, or “invasomes”, which can be briefly described as ultra-deformable vesicular carriers with upgraded transdermal penetration and increased permeability into the skin for the PSs compared with conventional liposomes [272][273][274][275]. On the other hand, recently reported aPDT micellar systems refer to micelles loaded with various hydrophobic PSs such as curcumin, BODIPY, porphyrins, hypocrellin A, or hypericin among others [106][276][277][278]. Furthermore, solid lipid NPs (SLN) composed of solid biodegradable lipids have been recently highlighted as delivery systems used for actual mRNA COVID-19 vaccines [279], but they also have been reported as transporters for curcumin for the treatment of oral mucosal infection [106]. Besides, we may also include nanoemulsions in this category of lipid-based nanovehicles since the latter conventionally involve lipids in the oily phase during the formulation process, with recent curcumin/curcuminoid nanoemulsions [106][280].
2.2.5. Polymer-Based Systems
In direct correlation with the above-mentioned lipid-based systems, polymer-based nanocarriers have been positioned as a logical extension with the objective to implement a “degree of freedom” in the synthesis flexibility while expanding the panel of building blocks available in the design of aPDT nanostructures. A categorization of polymeric systems for the delivery of PSs to therapeutically relevant sites can be done using as criteria either the nature of the polymer(s) involved or the type of polymeric (nano)structures. Thus, in the following, we will use a classification primarily based on the structure of the polymeric systems with differentiation between NPs (including hydrogels, biopolymers, and aerogels), polymersomes, polymeric micelles, dendrimers, fibers, and polymeric films and layers (including hybrid systems and nanocomposites). The nanostructure of polymeric systems is implicitly highly correlated to the molecular structure, i.e., composition (nature of the polymer(s), ratios, and distribution and amount of hydrophilic and hydrophobic moieties), charge, and size, as reviewed recently by Osorno et al. [281]. The refined control of these parameters enables the design and fine-tuning of specific polymeric nanostructures spanning from long-ranged ordered lamellar sheets, tubes, and fibers to oval or spherical particles, micelles, and polymersomes [281] (Figure 2C).
Conjugated Polymers as PSs or Polymer-Functionalized PSs
One of the easiest approaches reported to develop polymeric carrier systems is to enhance water solubility and biocompatibility of PS molecules through the use of functionalized polymers, by introducing the PSs in a post-modification reaction to a hydrophilic and/or biocompatible polymer, or to modify the PS with a polymerizable group (e.g., acrylate) to react as a monomer for further polymerization with suitable monomers. Other apt options are conjugated polymers incorporating a backbone with alternating double and single bonds which provide photodynamic and optical properties, and therefore might act as PSs themselves [282][283]. For example, poly[(9,9-bis{6′-[N-(triethylene glycol methyl ether)-di(1H-imidazolium)-methane]hexyl}-2,7-fluorene)-co-4,7-di-2-thienyl-2,1,3-benzo-thiadiazole] tetrabromide (PFDBT-BIMEG) is a conjugated polymer which affords salt bridges and electrostatic interactions with microorganisms; these interactions enable the simultaneous detection and inhibition of microorganisms [282]. Conjugated polymers were intensively investigated for the development of multifunctional NPs in antibacterial applications because of their generally low-toxicity toward eukaryotic cells, their flexibility, and their high potential to vehicle versatile therapeutic molecules [283]. Improved PSs based on conjugated polymers or polymerized PSs with antimicrobial photodynamic properties have been amply reported in recent years [284][285][286][287][288]. For example, Huang et al. demonstrated the efficiency and selectivity of polyethyleneimine-Ce6 conjugated in potential aPDT/PDI applications [289].
Other polymeric systems also show antimicrobial properties or preferential target infection sites and are thus suitable for aPDT applications when combined with PSs. For example, cationic polymers, which are known to be highly hydrophilic, can be used to target cell walls and thus microbial infections. Hence, such polymers are adequate for potential aPDT applications [290][291]. Exemplary amphiphilic or cationic poly(oxanorbornene)s doped with PSs, which exhibit pronounced antimicrobial activity (99.9999% efficiency) against E. coli and S. aureus strains, have been reported [292]. By contrast, anionic polymer particles and nanocarriers with negatively charged surfaces and membranes invade the reticuloendothelial system, which leads to elongated blood circulation times [293][294][295]. Polymers with large fractions of functional groups, such as the biopolymers mentioned above, can be easily modified and offer many loading sites for suitable PSs. Thus, among many suitable materials, hyperbranched and dendrimeric polymers are particularly promising candidates for PS nanocarriers.
Dendrimers
Dendrimers, dendrimeric polymers, or dendrons are highly ordered and highly branched polymers, which may form spherical three-dimensional structures with diameters typically ranging from 1–10 nm [296]. The dimensions of dendrimers are relatively small compared with other drug delivery systems, but as they consist of individual well-defined molecules, the drug loading can be subtly established with reproducibility. The incorporation or encapsulation of drugs may be achieved by covalent binding of the PSs or drug molecules to reactive functional groups along the dendrimeric structure [297][298]. Alternatively, the PS or drug molecule may act as a scaffold from which the dendrimer is synthesized. Finally, drug or PS encapsulation may occur inside the voids of the dendrimer [296][299][300]. Thus, dendrimers offer a substrate for PSs with many reaction sites, and controllable sterical and hydrophobic properties depending on the backbone of the dendrimer. Approaches using hyperbranched polymers for stimuli-responsive release of PSs, such as porphyrins, under acidic or reductive conditions, with improved targeting of bacterial sites have been reported, such as mannose-functionalized polymers by Staegemann et al. [301][302].
Polymeric NPs and Nanocomposites
In many cases, polymeric nano-systems are not clearly classified, but instead typically grouped under the terminology “polymeric NPs”, since the exact structure may not be fully characterized or considered less relevant with respect to the application efficiency. Polymeric NPs are commonly defined as physically or chemically crosslinked polymer networks with a size in the range of 1 to 1000 nm, but if not further defined may also include nanocapsules, such as polymersomes, micelles, chitosomes, and even highly branched polymers and dendrimers [303].
In aPDT, polymeric NPs may be either used to encapsulate PS molecules (loading with PSs), or built from inherent photoactive polymers acting as PSs [288]. Various PS molecules (e.g., RB, porphyrin derivatives, or curcumin) encapsulated in biocompatible polymeric NPs, such as polystyrene-, PEG-, polyester- (including poly-beta-amino esters), or polyacrylamide-based NPs, are widely used in aPDT/PDI approaches, as recently reported [304][305][306]. In addition to the role of nanovehicle, the polymeric particles may also help to address the lack of solubility in the biological medium from the PSs and/or reduce their toxicity, as reported among others by Gualdesi et al. [305][307]. For instance, polystyrene NPs with encapsulated hydrophobic TPP-NP(5,10,15,20-tetraphenylporphyrin) have been reported as nano-PS for efficient aPDI approaches towards multi-resistant bacteria [306]. In addition, biocompatible PLGA (polylactic-co-glycolic acid) NPs were employed to encapsulate curcumin, which could potentially serve as an orthodontic adhesive antimicrobial additive [307]. Alternatively, combinatory approaches such as polymeric NPs merging a polymeric PS, a photothermal polymeric agent, and poly(styrene-co-maleic anhydride) as dispersants have recently been reported for coupled aPDT/PTT nano-platforms in aqueous media [308][309]. Furthermore, Kubát et al. reported an increase in the stability of physically crosslinked polymeric NPs (polystyrene) to comparable nanocapsules (PEG- poly(ε-caprolactone) (PCL) micelles) equipped with identical PS [304].
The term polymeric NP also includes NPs obtained from natural polymers (e.g., chitosan and alginate), hydrogels, or aerogels. Hydrogels are chemically or physically crosslinked polymeric networks, which are able to absorb large amounts of water due to their pronounced hydrophilicity, but do not dissolve because of the crosslinking, whereas aerogels are formed by replacement of liquid with a gas resulting in low-density polymeric structures [310]. Recently, Kirar et al. reported PS-loaded biodegradable NPs fabricated from gelatin, a naturally occurring biopolymer, and hydrogel, for application in aPDT [311]. Moreover, also recently, some polymers such as Carbopol-forming hydrogel matrix entrapping PS molecules attracted attention because of their bio/muco-adhesive property, allowing a prolonged local PS delivery. Nevertheless, the viscosity of such systems can prevent the efficiency of PDT by decreasing the photostability and ROS production, thus calling for further optimizations [312][313]. Furthermore, the incorporation of hydrophobic PSs such as curcumin in polyurethane hydrogel has demonstrated to be an efficient PS release system [314].
Another suitable hydrogel for aPDT is chitosan, which is a polycationic biopolymer with good biocompatibility and antibacterial properties [315][316]. Indeed, cationic polymers, which can be antimicrobials by themselves such as chitosan and poly-Lysine, can assure a better recognition toward bacteria thus improving antimicrobial efficiency. Typically, polycationic chitosan may form nanogels or NPs in the presence of (poly)anionic molecules. Alternatively, another promising aPDT approach for the treatment of Aggregatibacter actinomycetemcomitans was recently reported [317][318], in which anionic PS indocyanine green was used to form PS-doped chitosan NPs. These NPs were shown to significantly reduce biofilm growth-related gene expression. Other similar approaches have also been reported, in which PS doped chitosan NPs showed enhanced cellular uptake and improved antimicrobial properties compared with free PS against different bacteria [197][319][320].
The above-mentioned approach has not only been implemented with NPs, but also in thin films and layers to create biocompatible and antimicrobial surfaces [321]. Indeed, in aPDT and especially aPDI, polymers are also used to create antimicrobial surfaces or matrices to embed PS units. Thus, combinatory approaches—using cellulose derivatives, alginates, chitosan, and other polymer-based materials as biocompatible substrates for PSs and nanocomposites to create photoactive antimicrobial surfaces—have recently been reported [322][323][324]. The development of such surfaces, and in particular antimicrobial membranes, is of considerable interest, especially in numerous and diverse fields in which providing hygienic and sterile surfaces is essential. As an illustration, Müller et al. reported polyethersulfone membranes doped with polycationic PS that provide antimicrobial properties for potential use as filter membranes in water purification or medicine [325]. Other recent distinctive systems for aPDI refer to self-sterilizing and photoactive antimicrobial surfaces made from (i) natural polymers such as chitosan doped with chlorophyll [321], (ii) “bioplastic” poly(lactic acid) surfaces coated with a BODIPY PS [326], or (iii) synthetic polyurethanes doped with curcumin and cationic bacterial biocides [321][327][328][329][330].
Alternatively, other combinatory approaches—in which polymeric nanocomposites embedding inorganic nanomaterials with relevant complementary features are conceived—have garnered attention in recent years. Examples include fullerenes or silver NPs that are incorporated as PSs into polymeric matrices [331], phthalocyanine-silver nanoprism conjugates [332], or mesoporous silica NPs loaded into polymer membranes [333]. Additionally, the embedding of zinc-based PS—such as Zn (II) porphyrin [334]—into polymer matrices to create antimicrobial polymers and polymeric surfaces for aPDI and possibly aPDT has also been reported, with pronounced antimicrobial activity against several bacteria strains and viruses [335][336]. Furthermore, protein-based approaches were developed as reported by Ambrósio et al. [337] and Silva et al. [338] using BSA (bovine serum albumin) NPs or BSA conjugated to PSs to improve solubility and biocompatibility.
Lastly, stimuli-responsive polymeric NPs are generally known to be sensitive to an internal or external stimulus (e.g., pH, temperature, light) and so of utmost interest for the controlled release of PSs for PDT and aPDT, whereas the stimuli-responsiveness depends on the structure and properties of the used polymer such as the assembly of polymer chains and linkages [339]. For example, Dolanský et al. reported light- and temperature-triggered ROS and NO release from polystyrene NPs for combinatory aPDT/PTT approaches [340]. Unlike micelles or polymersomes, crosslinked NPs have been reported to be thermodynamically stable, while stimulus-responsive behavior such as pH-responsive release of PSs has been more often achieved using self-assembled micellar or vesicular structures [304][341][342][343].
Polymersomes
Polymersomes are polymeric vesicles that resemble liposomes, which were previously described in Section 2.2.4 dedicated to lipid-based systems. Polymersomes are formed from amphiphilic copolymers, which self-assemble in aqueous media, resulting in capsules. The lumen of the polymersomes is filled with an aqueous medium and the wall comprises a hydrophobic interior with a hydrophilic corona on both inner and outer interfaces. Polymersomes typically form when the weight fraction of the hydrophilic parts (e.g., PEG in a PEG-b-poly(lactic acid) (PLA) block copolymers) comprises up to 20–40% of the polymer. They also form at comparatively large water fractions in the solvent shift method [344][345][346]. At higher weight fractions, the system tends to form micellar structures [281][347][348]. The hydrophilic and hydrophobic compartments inside the polymersomes enable the uptake and encapsulation of both hydrophobic and hydrophilic molecules [349][350][351][352], making polymersomes a relevant candidate for the development of advanced nanocarriers for PSs [353]. In aPDT and similar approaches, efficient delivery of the PSs to the targeted tissue is essential, not only to minimize toxic side effects and overcome low solubility in body fluids, but also to enable elongated circulation in the blood stream and prevent dimerization and quenching of the PSs. Li et al. reported that PSs encapsulated in polymeric nanocarriers exhibit an increased singlet oxygen 1O2 quantum yield compared with non-encapsulated PSs [293]; by contrast, non-encapsulated PSs may tend to aggregate and lose efficiency [293][354][355]. Additionally, most polymersomes offer certain advantages compared with the established liposome-based systems such as enhanced biocompatibility, lower immune response, controlled membrane properties, stimuli-responsive drug release, biodegradability, and higher stability, with those mainly resulting from the individual design of polymers and polymersomes for the anticipated applications [281][356].
The encapsulation of PSs into specifically designed nanosized polymersomes with stimulus-triggered release of PSs has been reported in recent years, including temperature, pH, and light-induced release of the PSs [281][357][358]. For instance, Lanzilotto et al. recently reported a system used for aPDT consisting of polymersomes of the tri-block-copolymer poly(2-methyl-2-oxazoline)-block-poly-(dimethylsiloxane)-block-poly(2-methyl-2-oxazoline) (PMOXA34–PDMS6–PMOXA34) encapsulating water-soluble porphyrin derivatives [357].
Polymeric Micelles
In contrast to polymersomes or liposomes, micelles are particles that contain a hydrophobic core. Typical micelles range between 10 to 100 nm in size. More specifically, polymeric micelles are formed of amphiphilic polymers, with a higher ratio of hydrophilic parts in the case of block copolymers compared with polymersomes [281][347][348]. They also form at comparatively small water fractions in the solvent shift method [344][345][346]. Due to their structures, micelles are implicitly used to encapsulate hydrophobic drugs or agents, such as PSs, to convey the latter to the target (e.g., cancer cells or microbial infections) while overcoming their low solubility in aqueous media [359]. Additionally, encapsulation may reduce the toxicity of the PSs [360][361]. When compared with polymersomes, one disadvantage is the lack of flexibility to encapsulate or transport both hydrophilic and hydrophobic molecules. On the other hand, as reported in the review by Kashef et al. [362], polymeric micelles are easier to produce, hence they are more cost-efficient than liposomes and potentially polymersomes, while providing similar applicable features [363][364]. Among examples, an aPDT system of polymeric micelles, fabricated from methoxy-PEG and PCL and loaded with the hydrophobic PS curcumin and ketoconazole for the PDI of fungal biofilms, has been reported with an increased water solubility and controlled release of the PSs on display [365]. Additionally, Caruso et al. recently reported the synthesis of thermodynamically stable PEG-PLA micelles for efficient aPDI of S. aureus, suggesting that this delivery system is promising in aPDI applications, which also reduces toxicity compared with pure PSs [276]. Moreover, a significant advantage of polymeric micelles compared with lipid-based micelles resides notably in the adjustability of properties by individual design of the polymer and membrane surface with respect to the selected application. Hence, the polymeric micelles can be equipped with target ligands and/or their morphology can be tuned to increase the cellular uptake of the micelle, PS, or other therapeutic agents in a specific tissue, such as in tumors [366]. Like polymersomes, polymeric micelles can be tuned to release drugs or collapse by application of external stimuli, such as light, temperature or pH [342][343].
Niosomes
Niosomes are closely related to liposomes. They are vesicular structures consisting of non-ionic surfactants, including polymers, and lipids such as cholesterol. The addition of lipids to the non-ionic surfactants leads to increased rigidity of the membrane and the vesicular structure. Niosomes range from 10 to 3000 nm in size, and also may include multi-layered systems typically consisting of more than one bilayer. Niosomes offer enhanced stability and biocompatibility compared with vesicular structures based on ionic surfactants [273]. Due to the bilayer structure and adjustability of the selected polymers for the applications of choice, they exhibit similar properties and offer similar advantages as polymersomes, and can be used for encapsulation of a variety of hydrophobic and hydrophilic molecules [273][343]. They may also be equipped with targeting ligands, such as folic acid that enhances uptake into cancer cells for PDT of cervical cancer [367]. A niosome-based system, using MB as encapsulated PS, has been reported for aPDT treatment of hidradenitis suppurativa [368].
Polymeric Fibers
Another approach to obtain PS carrier systems relies on polymeric or polymer-hybrid fibers with diameters in micro and nano range [369][370][371]. The fibers can either be loaded after formation with various PSs, such as cationic yellow or RB [372] incorporated into wool/acrylic blended fabrics to obtain antimicrobial properties, or they can be electrospun from PS containing polymer solution to form polymeric fibers loaded with PSs [373]. Since many polymers (such as nylon, cellulose acetate, or polyacrylonitrile) are used in the textile industry, those materials may offer a novel approach to create substrates or textiles with self-sterilizing and antimicrobial properties in the presence of visible light [371][374]. Furthermore, combinatory approaches using various NPs, such as magnetite NPs, to form polymeric fiber-based nanocomposites have shown some efficiency for antimicrobial photodynamic chemotherapy [375][376].
To conclude this Section 2.2, the list of above-mentioned aPDT nanosystems is non exhaustive and aims at providing an overview of the diversity and richness in the composition of aPDT nanomaterials. The next section focusing on “combinatorial strategies” partly overlaps with the description of aPDT systems, which further complicates the classification process. Moreover, a distinction must be settled between strict “mixtures” of components and “chemical combinations” of components in the development of aPDT treatments with possible synergistic behaviors.
3. Focus on Combinatory aPDT Approaches
Given its intrinsic characteristics, PDT is highly amenable to versatile combinations with other drugs, treatments, or modalities in view to potentiate therapeutic effects, which include enhanced efficacy, limitation of side effects, and reduction in the risk of resistance emergence. Proof-of-concept for various combinatory aPDT are thus being increasingly recorded, exploiting the additive/synergistic effects arising from single or multiple therapeutic species acting via different mechanistic pathways. The following part aims to review recent works following such strategies.
3.1. “Basic” aPDT Combinations
3.1.1. Combination of Several PSs
The simplest combinatory aPDT approach probably consists in combining two or more PSs in a single treatment. Thus, besides aPDT making use of a single PS, aPDT may rely on the simultaneous use of several PSs in an attempt to obtain additive or synergistic antimicrobial effects. The PSs combined may exhibit different photophysical characters showing complementarities. For example, carboxypterin-based aPDT upon sunlight irradiation demonstrates a significant planktonic bacterial load reduction [377]. However, eradication of biofilm formation needs a PS concentration 500 times higher than assays performed with planktonic forms. When combined with MB, Tosato et al. showed that reasonable concentrations of both PSs exert synergistic effect on both biofilm and planktonic MDR bacteria [378]. In other studies, alternative dual-PSs aPDT systems have shown even better antibacterial and antibiofilm properties [379][380].
3.1.2. Addition of Inorganic Salts
The combination of PSs with inorganic salts can modulate the PDT effectiveness, potentiating or inhibiting the antimicrobial activity through the production of additional reactive species or quenching 1O2 [381]. As an example, azide sodium can modulate aPDT effectiveness by promoting or inhibiting the binding of bacteria with PSs, depending on lipophilicity of the latter. Indeed, azide sodium is a 1O2 quencher, but can also produce highly reactive azide radicals, via electron transfer from PS at the excited state. This has been reported with many phenothiazinium dyes and also fullerenes [77][382][383]. Other salts can amplify the bacterial killing mediated by MB-PDT such as potassium iodide (KI), a very versatile salt, involved in the generation of short-lived reactive iodine radicals (I•/I2•−) [384][385]; similar findings have been obtained when using KI with cationic BODIPY derivatives [386] or porphyrin–Schiff base conjugates bearing basic amino groups [387]. Potassium thiocyanate and potassium selenocyanate could also form reactive species, such as the sulfur trioxide radical anion and selenocyanogen (SeCN)2, respectively [388]. In addition, interactions between PSs and target microorganisms could be improved thanks to inorganic salts. For example, calcium and magnesium cations can modulate the electrostatic interaction between PSs and bacterial membranes [389]. It is worth noting here that most of the above-mentioned studies were conducted under in vitro settings. While inorganic salts can be useful to enhance in vitro or ex vivo aPDT effects, the use of such additives in animals or human beings would need to be carefully examined, considering the dose to be used and potential concomitant side effects.
3.2. Combinations of aPDT with Other Antimicrobial Drugs or Antimicrobial Therapies
Various classes of antimicrobial drugs or other antimicrobial therapies, which action does not depend on light, have been considered with regard to their aPDT compatibility.
3.2.1. Antibiotics
Antibiotherapy is an obvious complementary therapy to aPDT, which may allow obtaining stronger antimicrobial effects and/or restore antibiotic susceptibility. Combinations of PSs with antibiotics have been investigated in numerous studies addressing various infectious diseases, such as skin and mucosal infections as recently reviewed [390]. Aminosides are the most common antibiotics used in combination with a PS. For instance, kanamycin, tobramycin, and gentamicin combined respectively to RB, porphyrin, and 5-Alanine have been reported, showing potent effects against bacteria of clinical interest, notably by improving biofilm clearance and reducing microbial loads [391][392][393][394][395]. In addition, combinations with other antibiotic classes, such as nitroimidazoles (e.g., metronidazole) or glycopeptides (e.g., vancomycine), also showed some effect against biofilms of Fusobacterium nucleatum and Porphyromonas gingivalis [396], as well as S. aureus [397]. Further, PSs can also be useful to combine with antibiotics in order to restore the susceptibility of bacteria to the latter, notably to last-resort antibiotics. As a recent example, Feng et al. reported the photodynamic inactivation of bacterial carbapenemases, both restoring bacterial susceptibility to carbapenems and enhancing the effectiveness of these antibiotics [398]. However, all combinations of PSs and antibiotics may not be effective, since in some cases antibiotics can have an antagonistic effect on PS activity [399]. Finally, it is noteworthy that some antibiotics can act themselves as PSs; Jiang et al. indeed reported light-excited antibiotics for potentiating bacterial killing via ROS generation [400].
3.2.2. Antifungals
The combination of PSs with antifungals is a powerful approach to thwart growing antifungal resistance, especially to fluconazole, which is commonly observed in Candida albicans strains [401]. Quiroga et al. showed that sublethal photoinactivation mediated by a tetracationic tentacle porphyrin allowed to reduce the MIC of fluconazole in C. albicans [402]. Nystatin, a common antifungal used to prevent and treat candidiasis, was combined with photodithazine-based PS and red light in C. albicans-infected mice. The combined therapy reduced the fungal viability and decreased the oral lesions and the inflammatory reaction [29]. Moreover, other antifungals (e.g., terbinafine, itraconazole and voriconazole) combined to ALA reported promising results. These combinations could be alternative methods for the treatment of refractory and complex cases of chromoblastomycosis [403][404].
3.2.3. Other Antimicrobial Compounds
Many other antimicrobial compounds may be used in combination with PSs. Antimicrobial peptides (AMPs) are oligopeptides (commonly consisting of 10–50 amino acids) with high affinity for bacterial cells thanks to an overall positive charge. Their antimicrobial spectrum can be modulated through variation of their amino acids sequence. AMPs encompass a large set of natural compounds that may be used in aPDT. For example, de Freitas et al. reported that sub-lethal doses of PSs (either Ce6 or MB) and aurein peptides (either aurein 1.2 monomer or aurein 1.2 C-terminal dimer) were able to prevent biofilm development by E. faecalis [405]. In another study, a membrane-anchoring PS, named TBD-anchor, demonstrated both bacterial membrane-anchoring abilities and ROS production [406]. In a similar way to peptide therapy, LPS-binding proteins were used to improve the contact of PSs with the cytoplasmic membrane of bacteria. For instance, the antibacterial efficacy of a complex consisting of a combination of RB with the lectin concanavalin A (ConA) was demonstrated in a planktonic culture of E. coli; ConA-RB conjugates increased membrane damages and enhanced the RB efficacy up to 117-fold [407]. In addition, coupling pump efflux inhibitors, such as CCCP EPI (carbonyl cyanide m-chlorophenylhydrazone), to PSs was also investigated. This highlighted the interest to combine PSs with molecules acting on targets susceptible to induce resistance modulations [57]. Other antimicrobial molecules may be good candidates to be used in aPDT strategies. For example, quinine used in combination with antimicrobial blue light was shown efficient to photo-inactivate MDR P. aeruginosa and A. baumannii [408]. Some cationic molecules, such as cationic lipids, can have a good affinity for bacterial cell membrane and a good antibacterial activity [111][409]. Thus, PS-amphiphiles conjugates would also deserve to be investigated for aPDT applications.
3.2.4. Viral NPs and Phagotherapy
In recent years, viral NPs (VNPs) deriving from phages, animal, or plant viruses have been proposed as biological vehicles for delivery of PSs. Such carriers can exhibit a series of advantageous properties including natural targeting, easy manufacture and good safety profile [410]. Compared with nanomaterials used as PS carriers, VNPs are natural protein-based NPs that may display higher biocompatibility and tissue specificity. In addition, VNPs can be tuned through genetic and synthetic engineering with appropriate biological and chemical modifications (e.g., surface decoration). Alternatively, the combination of aPDT with so-called phagotherapy may be useful for various reasons, following different strategies. One study suggests that, following a PDT treatment, ROS damages can cause quorum sensing and virulence pathway alterations rendering micro-organisms more susceptible to other therapies. Among these, phagotherapy is known to be modulated by multiple virulence factors [411]. Another approach can consist of conjugating phages with PSs, in order to improve interaction/delivery of the latter into target microorganisms. For example, Dong et al. showed that a phage carrying the chlorophyll-based PS pheophorbide efficiently induced apoptosis in C. albicans, thus demonstrating the potential of phototherapeutic nanostructures for fungal inactivation [412]. However, such an approach has to be prudently considered regarding the occurrence of phage resistance already reported in numerous investigations [413].
3.3. Combinations of aPDT with Other Light-Based Treatments
Multiple modalities entirely controlled by light stimuli may be combined with aPDT in multifunctional antimicrobial treatments. The following part reports some very recent studies illustrating multiple light-based antimicrobial strategies combined to operate independently, with potential additive or synergistic effects and without reciprocal interferences. This may be obtained with a single PS displaying such multiple properties and/or through combination of PSs with other light-activatable compounds exhibiting complementary properties.
3.3.1. aPDT and Photothermal Therapy
Photothermal therapy (PTT) is a local treatment modality relying on the property of a PS to absorb energy and convert it into heat upon stimulation with an electromagnetic radiation, such as radiofrequency, microwaves, near-infrared irradiation, or visible light. The localized hyperthermia can lead to various damages resulting in microbial inactivation in the treatment area. Following this principle, ruthenium NPs have been used for PDT/PTT dual-modal phototherapeutic killing of pathogenic bacteria [414]. Moreover, GO demonstrated antibacterial effect against E. coli and S. aureus as a result of both PDT and PTT effects following irradiation with ultra-low doses (65 mW/cm2) of 630 nm light [415]. Furthermore, combination of sonodynamic, photodynamic, and photothermal therapies with an external controllable source recently reported against breast cancer [416] may also show promising applications for treating bacterial infection. Mai et al. reported a FDA-approved sinoporphyrin sodium (DVDMS) for photo- and sono-dynamic therapy in cancer cells and photoinactivation of S. aureus strains, in in vitro and in vivo models. However, no bacterial sonoinactivation by DVDMS was obtained [417].
3.3.2. aPDT and NO Phototherapy
Combination of aPDT with NO phototherapy is gaining increasing interest for antimicrobial applications [418]. For instance, light-responsive dual NO and 1O2 releasing materials showed phototoxicities against E. coli [340][419]. More recently, Parisi et al. developed a molecular hybrid based on a BODIPY light-harvesting antenna producing simultaneously NO and 1O2 upon single photon excitation with green light for anticancer applications; according to the authors, this system may also act as an effective PS and NO photodonor antibacterial agent [420].
3.3.3. aPDT and Low Laser Therapy
Photobiomodulation (PBM), also called low-level laser therapy, is a non-destructive process that may alleviate pain and inflammation or promote tissue healing and regeneration. The use of this method coupled to aPDT is a very recent approach. A concomitant use of aPDT and PBM was reported as an adjunct treatment for palatal ulcer [421]. In a clinic-laboratory study, aPDT and PBM showed similar improvement in gingival inflammatory and microbiological parameters compared with conventional treatment [422]. More recently, some benefits of this combined therapy were reported such as the modulation of inflammatory state, pain relief, and acceleration of tissue repair of patients contracting herpes simplex labialis virus or orofacial lesions in patients suffering from COVID-19 [423][424][425].
3.4. Coupling of aPDT with Other Physical Treatments
3.4.1. aPDT and Sonodynamic Therapy
Sonodynamic therapy (SDT), combining so-called sonosensitizers (SS) and ultrasounds (US) is a relatively new approach for treating microbial infections [426][427]. The ultrasonic waves have the property to induce a cavitation phenomenon thus enhancing the efficacy of combined antimicrobial treatments. The rationale for combining PDT with SDT relies on specific advantages of the latter, notably, a deeper propagation of US into the tissue than light; therefore, PDT/SDT may be used to treat deeper lesions in vivo, alleviating the limitations of light propagation and delivery presented by aPDT [428][429]. An approach combining PDT and SDT, called sonophotodynamic therapy (SPDT), has been reported to improve microbial inactivation compared with individual aPDT or SDT [430]. Because of the complicated system of SPDT, its mechanisms have not been clearly revealed yet. Some studies have demonstrated that sonoporation mechanism induced by US improves the transfer of large molecules into the bacteria by forming transient pores. Moreover, US waves could potentiate the microorganisms dispersion in the medium resulting in (i) a better biodisponibility of therapeutic agent and light diffusion and (ii) a reduction of microbial aggregation and networks, such as biofilm [430][431]. The mention of a new class of PS characterized by the dual ability to be activated by both US and light, for SPDT application, has been questioned. Indeed, Harris et al. recently suggested that this specific PS/SS class could be useful for antimicrobial application – beside previously reported anticancer application – with initial investigation using chlorins as dual PS/SS agent [432]. Since this study by Harris et al., a few dual-activated PS/SS have been described that would warrant further investigations [433][434].
3.4.2. aPDT and Electrochemotherapy
Electrochemotherapy, also called pulsed electromagnetic fields (PEMFs) or electropermeabilization, is a method consisting in applying an electrical field to cells in order to enhance their permeability to therapeutic molecules (often chemical drugs or DNA). Combination of PDT with electrochemotherapy has been used many times to treat cancer diseases [60][435][436]. One study showed that, compared with aPDT used alone, hypericin combined with electrochemotherapy allows to achieve more than 2 to 3 log10 CFU reduction in E. coli and S. aureus, respectively [437]. To our knowledge, no other study combining electrochemotherapy with aPDT was recorded since then. However, combination of aPDT and a cell-permeabilization technique with a controllable toxicity degree may be highly relevant since most PSs can act in extracellular medium without having a specific target. Accordingly, electrochemotherapy may allow boosting aPDT activity by promoting PSs internalization into target microorganisms.
3.5. aPDT and Other Antimicrobial-Related Therapies
Immunotherapeutic effects may be obtained as a result of PDT itself or due to other treatments used in combination. For instance, Schiff base complexes with differential immune-stimulatory and immune-modulatory activities were reported efficient to eliminate both Gram(+) and Gram(−) bacteria. Furthermore, upon photoactivation, these complexes blocked the production of the inflammatory cytokine TNFα, thereby allowing to treat at once bacterial infections associated with damaging inflammation [438]. One recent study proposed the first application of antimicrobial photoimmunotherapy (PIT) by developing a PS-antibody complex, selective to the HIV antigen anchored to the infected cell membranes [439]. Such an approach supports the therapeutic applicability of PDT against antimicrobial infections, especially those mediated by intracellular pathogens. In addition, photodynamic therapy using PSs at sub-lethal concentrations may exhibit interesting properties for inflammatory and infectious conditions [440]. It was shown effective to alter immune cell function and alleviate immune-mediated disease, to hasten the process of wound healing, and to enhance antibacterial immunity. PDT thus appears as a promising therapeutic modality in infectious and chronic inflammatory diseases such as inflammatory bowel disease and arthritis.
4. Other aPDT Perspectives: New Strategies to Efficiently Target Bacteria
Irrespective of the biomedical applications, achieving a precise targeting is crucial to guarantee both efficiency and specificity. For anticancer PDT, many targeting studies have been done, notably for evaluating PSs covalently attached to molecules having affinity for neoplasia or ligands for receptors expressed on tumors. By this way, PSs may be chosen considering primarily their ability to achieve high PDT effects rather than depending on their intrinsic targeting properties. Following the same rationale, aPDT-based combinatory systems can be developed, benefiting from earlier studies performed in multimodal oncology [441].
4.1. Aggregation-Induced Emission (AIE) Luminogens
AIE luminogens exhibit, in the aggregated state, nonradiative decay and show bright fluorescence due to the restriction of intramolecular motions [17]. Recently, their interests for antimicrobial applications have been reported, showing the possibility to simultaneously perform detection and image-guided elimination of bacteria for theranostics applications [442]. In comparison with classical PSs, AIE luminogens in an aggregated state do not exhibit self-quenched fluorescence and ROS production is better. For instance, Gao et al. reported a tetraphenylethylene-based discrete organoplatinum(II) metallacycle electrostatically assembled with a peptide-decorated virus coat protein. This assembly showed strong membrane-intercalating ability, especially in Gram(−) bacteria, and behaved as a potent AIE-PS upon light irradiation [443].
4.2. Photochemical Internalization (PCI)
PCI may be used to enhance cell internalization of diverse macromolecules. It consists of PDT-induced disruption of endocytic vesicles and lysosomes improving the release of their payloads into the cytoplasm of target cells. Although most PCI applications relate to cancer treatments, PCI could be extended to treat intracellular infections by delivering antimicrobials into infected cells [444]. For instance, Zhang et al. reported PCI as an antibiotic delivery strategy allowing to enhance cytoplasmic release of Gentamicin, to counter intracellular staphylococcal infection in eukaryotic cells and in zebrafish embryos [445].
4.3. Genetically-Encoded PSs
Internalization of PSs inside target microorganisms could be facilitated thanks to their conjugation with adjuvants, as mentioned before. Alternatively, it could be possible to use genetically-encoded ROS-generating proteins (RGPs), also called genetically-encoded PSs. Such an approach represents a powerful way to “completely localize” PSs inside target microorganisms for highly specific antimicrobial phototoxicity. Furthermore, in situ production of RGPs allows to enhance interaction with intracellular targets and better control the biodistribution of PSs, while limiting side-effects for the host tissues and environments [446]. To date, two groups of RGPs have been reported; those that belong to the green fluorescent protein (GFP) family and form their chromophores auto catalytically, and those that use external ubiquitous co-factors (flavins) as chromophores [447]. For example, Endres et al. compared eleven light-oxygen-voltage-based flavin binding fluorescent proteins and showed that most were potent PSs for light-controlled killing of bacteria [448].
4.4. pH-Sensitive aPDT
Some studies have reported smart photoactive systems consisting in PSs assembled in nanoconjugates with acid-cleavable linkers. For instance, Staegemann et al. described porphyrins conjugated with acid-labile benzacetal linkers and demonstrated the cleavage of the active PS agents from the polymer carrier in the acidic bacterial environment [301]. In addition, photoacids may be useful to design pH-sensitive aPDT systems. Upon light irradiation, such agents promote the spatial and temporal control of proton-release processes and could provide a way to convert photoenergy into other types of energy [449]. Thus, proof-of-concept was reported for the use of reversible photo-switchable chemicals as antimicrobials inducing MDR bacteria photoinactivation mediated by the acidification of intercellular environment [450]. To our knowledge, no studies have yet reported the potential of photoacids in combination with aPDT systems. However, some pH-sensitive PSs can induce remarkable variations of antimicrobial photoinactivation levels under different environmental pH [451]. These observations suggest the potential of photoacids as PDT potentiators for enhanced antimicrobial applications.
4.5. DNA Origami as PS Carriers
The quite recent development of DNA origami based on well-established DNA nanotechnology can serve as an excellent scaffold for the functionalization with different kinds of molecules and could be a powerful tool, as described by Yang at al., to study in a real-time conditions the assemble/disassemble of photo-controllable nanostructures [452]. Oligonucleotides organized as DNA origami could thus be used as PS-carrying nanostructures featuring numerous and dense intercalation sites. In addition, the tightly packed double helices can avoid the degradation by DNA hydrolases in the cellular environment. For instance, Zhuang et al. reported the uptake in tumor cells of a PS-loaded DNA origami nanostructure where it generated free radicals, releasing PSs due to DNA photocleavage, and induced cell apoptosis [453]. To our knowledge, such an approach has not yet been investigated for antimicrobial purposes.
5. Discussion
Antimicrobial PDT has the potential to fight against a wide spectrum of infective agents, including those resistant to conventional antimicrobials, under non-clinical and clinical settings. Rather than replacement, aPDT may be a complementary approach to reduce the use of current, especially last resort, antimicrobials. This review aims to give a non-exhaustive overview of the diversity and richness of synthetic, natural, or hybrid single PSs and aPDT nanosystems that were recently reported, with respect to their specific advantages, limitations, and possible evolutions. It is noteworthy that many systems and strategies primarily developed for anticancer PDT have been or could be applied—per se or following adaptations—to antimicrobial applications.
Beyond the “chemical space” that can be explored with individual PSs, versatile combinations with other compounds can allow the design of multimodular/multimodal systems. Along this line, the various PSs available may be considered as “basic ingredients”. Apart from offering alternative possibilities for overcoming the most common limitations of PSs (i.e., solubility, delivery, and specificity), reasons for implementing PSs in complex systems can be related to (i) the ability to target several types of pathogens at once (extended antimicrobial spectrum), (ii) synergistic antimicrobial effects, (iii) reduction in the dose of each combined component, (iv) beneficial effects in severe poly-pathogenic infections, and (v) reduction in the risk of resistance emergence.
In addition to the many possible variations concerning PSs, optimizations can also consider other critical parameters in PDT, namely light irradiation and oxygenation. The latter was considered for a long time as an indispensable component. However, control of the oxygen level in the aPDT system is questionable. Indeed, additional oxygen-independent phototoxic mechanisms have been reported, for example with psoralens, which can produce more effective aPDT without oxygen [16]. Furthermore, recent studies suggest that various strategies could be used to reduce or bypass the limitations of oxygen and light supplies (read below). All combined, optimizations targeting not only the PSs, but also light irradiation and oxygen supply, could allow to evolve toward integrative, highly sophisticated, antimicrobial photodynamic therapy.
Light irradiation and its various modalities have been reviewed in depth by different authors [13][454][455][456]. Typically, the irradiation in PDT occurs in the UV (200–400 nm) or in the visible light (400–700 nm) with a power of ≤ 100 mW [455]. However, the low light-penetration depth (around mm) and possible occurrence of tissue photodamage limit the applicability of this spectral range for PDT. This can be circumvented by application of a near-infrared (NIR) irradiation (750–1100 nm), particularly via a two-photon excitation, which is emerging for PDT applications [208][457]. Being a third-order nonlinear optic phenomenon and corresponding to the simultaneous absorption of two photons with half the resonant energy, it allows deeper penetration in biological tissues (around 2 cm), lower scattering losses, and a three-dimensional spatial resolution [458]. Light sources are also constantly improving. Laser is an exceptional source of radiation, capable of producing extremely fine spectral bands, intense, coherent electromagnetic fields ranging from NIR to UV. In comparison, LEDs feature other advantages, notably the possibility to be arranged in many ways, in large quantity, for irradiating wide areas while inducing negligible heating [454][455].
Considering that light-emitted sources can become a brake to any PDT applications, several promising options have been envisaged quite recently. Notably, Blum et al. have identified a series of “self-exiting way” that allows to abolish the need of light to achieve efficient PDT [459]. Among them, chemiluminescence was extensively investigated by using luminol or luciferase energy transfer to induce a chemiexcitation of PS as antibacterial therapeutics [460]. In addition, other methods may be based on Cherenkov radiation, which occurs when an emitted charged particle, such as an electron, moves with high speed through a dielectric medium, such as water. Thus, the polarization of electrons in the medium produces electromagnetic waves in the visible wavelengths that could activate PDT reaction. Another way to induce Cherenkov radiation is to use radioactive isotopes with high beta emissions (e.g., 18F, 64Cu, or 68Ga) as an electronic excitation source [459]. The relevance of such approaches for the design of a self-induced aPDT system remains to be evaluated. In addition, another external source of excitation, such as microwaves, could activate photoactive molecules such as Fe3O4 when they are complexed with carbon nanotubes. This was recently evaluated by Qiao et al. for treating MRSA-infected osteomyelitis [23]. Furthermore, X-ray as an activated source can also facilitate the activation of the PDT system by transferring energy harvested from X-ray irradiation to the PS used [461]. Kamanli et al. compared pulse and superpulse radiation modes, showing that the latter is more effective to produce 1O2 and S. aureus eradication than the former [462].
Oxygen is also a critical limiting factor that determines PDT efficiency, especially in poorly oxygenated environments. However, it is noteworthy that PDT can be achieved in cancers that typically feature a low oxygenation rate. To alleviate the possible limitations of oxygen supply in PDT systems, several strategies have also been recently considered. One is based on catalase grafting to achieve oxygen self-sufficient NPs in order to convert H2O2 into available dissolved oxygen in the tumor environment. The abundant ROS in tumors compared with normal tissues provide a coherent substrate for catalase and thus allows an improvement of PDT activity [463]. Some multifunctional nanomaterials, called nanozymes, can also be used in combination with PSs to achieve a catalase-like activity supplying an oxygen source for the PS functioning [464][465]. In addition, it was also reported that noble metal NPs – such as Ru, Pt, and Au NPs – exhibit catalase-like nanozyme activities [466]. The use of catalases or nanozymes may have the crucial role of oxygen helpers in aPDT for treating deep infections.
The possibilities to design combinatorial aPDT strategies seem unlimited. Those presented in this review partly overlap with the description of aPDT systems, showing the complexity of any classification process. Awareness and caution may be raised about some sort of “paradox” or “dilemma”; indeed, while the seemingly boundless collection of chemical options and modular tools to develop nanoscale aPDT therapeutics implicitly defines extensive design flexibility, it staggeringly complicates and bewilders at the same time the optimization process aiming to compare, rationalize, and identify the “best” option for each application. Along this line, data concerning structure-activity relationships are usually missing, with not many studies yet dedicated to this matter [52][65][75][467]. Similar to the CLSI guidelines defined for antibiotics, uniform research methodologies would be useful to assay aPDT systems under well-defined standards and guidelines, considering notably (i) illumination settings, (ii) positive controls [468], (iii) microorganisms and cell lines relevant for a given application, and (iv) assessment of antimicrobial efficacy; this would guarantee better-conducted preclinical and clinical trials of aPDT systems used as mono or combinatory therapy. Furthermore, a public database compiling the efficacy/side effects of various systems would also be useful, facilitating meta-analyses for delineating (quantitative) structure/activity relationships and computational simulations [469].
Finally, in view of translation to clinical practice, a series of precautions and potential limitations must also be considered, especially when dealing with combinatory strategies. Beside possible reciprocal interferences (antagonisms) between combined partners, safety and specificity parameters must also be carefully examined. One main advantage of aPDT is the possibility to control the production of ROS thanks to the use of nontoxic PSs triggered with inducers (specific light and free oxygen) and/or enhancers. The vast majority of PSs are considered safe and dark cytotoxic side effects toward non-target eukaryotic cells are rarely reported. However, in many cases, more studies are needed for examining—beyond potential side-effects—other parameters such as bioavailability, biodispersion, persistence in host cells and body, and elimination pathways. With regard to combinatory strategies, it is noteworthy that studies conducted to date were mostly based on in vitro evaluation or using animal models bearing well-defined sites of infection. Thus, much more data in preclinical and clinical settings are required to support the actual potential of such strategies. Moreover, considering that many microbial infections are systemic, the use of modalities such as sonodynamic therapy or electrochemotherapy is at present not realistically feasible. Therefore, in spite of significant progress and real promise, further important work/innovations are needed to effectively broaden the range of infectious conditions that could be treated via aPDT approaches. Lastly, cost effectiveness of multiplexed aPDT therapies over monomodal conventional antimicrobial agents is another crucial point to be considered in view of clinical applications. Potent, but too expensive solutions may fail to be used in practice, especially in under-developed countries where conventional antimicrobials will continue to be used, allowing microorganisms to increase in resistance.
6. Concluding Remarks
In conclusion, aPDT is a versatile approach that tends to evolve from quite a simple method to reach much higher degrees of complexity, with several expected advantages, but also possible drawbacks or undesirable effects. Among the latter, any direct/indirect impact on AMR should be more thoroughly considered. It is noteworthy that aPDT clinical trials conducted to date evaluated quite simple PSs. Any increase in the complexity of therapeutic systems would lead to an increase in difficulty before being able to reach clinical applications. The development of effective and safe aPDT treatments requires expertise in many fields of research, including biology (microbiology, cell biology, biochemistry, pharmacology), chemistry, physics (optical physics), and engineering. This is even more the case with combinatory strategies involving different modalities as reviewed in this article. Translation to practical applications also implies strong collaborations with the different sectors of health care and pharmaceutical companies. In these conditions, aPDT and its many therapeutic combinations could become a frontline routine treatment to fight against microorganisms possibly responsible for the next healthcare crises [37].
7. Abbreviations
| AIE | aggregation-induced emission |
| ALA | alanine |
| AMP | antimicrobial peptide |
| AMR | antimicrobial resistance |
| aPDT | antimicrobial PDT |
| ConA | concanavalin A |
| CNTs | carbon nanotubes |
| Ce6 | chlorin e6 |
| CFU | colony forming unit |
| e− | electron |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. |
| IC | internal conversion |
| ICG | indocyanine green |
| ISC | inter-system crossing |
| GO | graphene oxide |
| H2O2 | hydrogen peroxide |
| HO● | hydroxyl radical |
| MB | methylene blue |
| MDR | multidrug resistance |
| MRSA | methicillin resistant S. aureus |
| MOF | metal organic framework |
| NIR | near infrared |
| NO | nitic oxide |
| NP | nanoparticle |
| O2 | dioxygen |
| O2●− | superoxide anion radical |
| 1O2 | singlet oxygen |
| 3O2 | ground state molecular oxygen |
| PDT | photodynamic therapy |
| PS | photosensitizer |
| PS•− | PS radical anion |
| 1PS | PS in the ground state |
| 1PS* | PS in a first excited singlet state |
| 3PS* | PS in a triplet excited state |
| PEG | poly(ethylene glycol) |
| PCI | photochemical internalization |
| PCL | poly(ε-caprolactone) |
| PLA | poly(lactic acid) |
| pSi | porous silicon |
| PTT | photothermal therapy |
| QD | quantum dot |
| R | reduced molecule |
| R●+ | oxidized molecule |
| RB | rose bengal |
| ROS | reactive oxygen species |
| SS | sonosensitizer |
| SDT | sonodynamic therapy |
| SPDT | sonophotodynamic therapy |
| SPION | superparamagnetic iron oxide NP |
| TB(O) | toluidine blue |
| UCNP | upconversion NP |
| UV | ultraviolet |
| WHO | world health organisation |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13121995
References
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175.
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539.
- Antoñanzas, F.; Goossens, H. The Economics of Antibiotic Resistance: A Claim for Personalised Treatments. Eur. J. Health Econ. 2019, 20, 483–485.
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66.
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910.
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80.
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55.
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348.
- Baier, J.; Maier, M.; Engl, R.; Landthaler, M.; Bäumler, W. Time-Resolved Investigations of Singlet Oxygen Luminescence in Water, in Phosphatidylcholine, and in Aqueous Suspensions of Phosphatidylcholine or HT29 Cells. J. Phys. Chem. B 2005, 109, 3041–3046.
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The Role of Singlet Oxygen and Oxygen Concentration in Photodynamic Inactivation of Bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228.
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39, 3181.
- Foote, C.S. Definition of Type I and Type II Photosensitized Oxidation. Photochem. Photobiol. 1991, 54, 659.
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589.
- Baptista, M.d.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919.
- Raphaëlle Youf; Max Müller; Ali Balasini; Franck Thétiot; Mareike Müller; Alizé Hascoët; Ulrich Jonas; Holger Schönherr; Gilles Lemercier; Tristan Montier; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995, 10.3390/pharmaceutics13121995.
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53.
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. WIREs Nanomed. NanoBiotechnol. 2019, 11, e1560.
- Garcez, A.S.; Kaplan, M.; Jensen, G.J.; Scheidt, F.R.; Oliveira, E.M.; Suzuki, S.S. Effects of Antimicrobial Photodynamic Therapy on Antibiotic-Resistant Escherichia Coli. Photodiagn. Photodyn. Ther. 2020, 32, 102029.
- Paziani, M.H.; Tonani, L.; de Menezes, H.D.; Bachmann, L.; Wainwright, M.; Braga, G.Ú.L.; von Zeska Kress, M.R. Antimicrobial Photodynamic Therapy with Phenothiazinium Photosensitizers in Non-Vertebrate Model Galleria Mellonella Infected with Fusarium Keratoplasticum and Fusarium Moniliforme. Photodiagn. Photodyn. Ther. 2019, 25, 197–203.
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-Mediated Photodynamic Therapy for the Treatment of Oral Infections—A Review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415.
- Cieplik, F.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Hiller, K.-A.; Maisch, T.; Karygianni, L. Antimicrobial Photodynamic Therapy as an Adjunct for Treatment of Deep Carious Lesions—A Systematic Review. Photodiagn. Photodyn. Ther. 2017, 18, 54–62.
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial Photodynamic Therapy—a Promising Treatment for Prosthetic Joint Infections. Lasers Med. Sci. 2018, 33, 523–532.
- Qiao, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; Li, Z.; et al. Treatment of MRSA-Infected Osteomyelitis Using Bacterial Capturing, Magnetically Targeted Composites with Microwave-Assisted Bacterial Killing. Nat. Commun. 2020, 11, 4446.
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026.
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202.
- Cabrini Carmello, J.; Alves, F.; Basso, F.G.; de Souza Costa, C.A.; Tedesco, A.C.; Lucas Primo, F.; Mima, E.G.d.O.; Pavarina, A.C. Antimicrobial Photodynamic Therapy Reduces Adhesion Capacity and Biofilm Formation of Candida Albicans from Induced Oral Candidiasis in Mice. Photodiagn. Photodyn. Ther. 2019, 27, 402–407.
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic Therapy of Oral Candida Infection in a Mouse Model. J. Photochem. Photobiol. B 2016, 159, 161–168.
- Leanse, L.G.; Goh, X.S.; Dai, T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2020, 52, 569–575.
- Janeth Rimachi Hidalgo, K.; Cabrini Carmello, J.; Carolina Jordão, C.; Aboud Barbugli, P.; de Sousa Costa, C.A.; Mima, E.G.d.O.; Pavarina, A.C. Antimicrobial Photodynamic Therapy in Combination with Nystatin in the Treatment of Experimental Oral Candidiasis Induced by Candida Albicans Resistant to Fluconazole. Pharmaceuticals 2019, 12, 140.
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagn. Photodyn. Ther. 2020, 31, 101774.
- Liu, Z.; Tang, J.; Sun, Y.; Gao, L. Effects of Photodynamic Inactivation on the Growth and Antifungal Susceptibility of Rhizopus Oryzae. Mycopathologia 2019, 184, 315–319.
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747.
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612.
- Mohr, H.; Knüver-Hopf, J.; Gravemann, U.; Redecker-Klein, A.; Müller, T.H. West Nile Virus in Plasma Is Highly Sensitive to Methylene Blue-Light Treatment. Transfusion 2004, 44, 886–890.
- Ohtsuki, A.; Hasegawa, T.; Hirasawa, Y.; Tsuchihashi, H.; Ikeda, S. Photodynamic Therapy Using Light-Emitting Diodes for the Treatment of Viral Warts. J. Dermatol. 2009, 36, 525–528.
- Zhang, W.; Zhang, A.; Sun, W.; Yue, Y.; Li, H. Efficacy and Safety of Photodynamic Therapy for Cervical Intraepithelial Neoplasia and Human Papilloma Virus Infection. Medicine 2018, 97, e10864.
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320.
- Zarubaev, V.V.; Belousova, I.M.; Kiselev, O.I.; Piotrovsky, L.B.; Anfimov, P.M.; Krisko, T.C.; Muraviova, T.D.; Rylkov, V.V.; Starodubzev, A.M.; Sirotkin, A.C. Photodynamic Inactivation of Influenza Virus with Fullerene C60 Suspension in Allantoic Fluid. Photodiagn. Photodyn. Ther. 2007, 4, 31–35.
- Abada, Z.; Cojean, S.; Pomel, S.; Ferrié, L.; Akagah, B.; Lormier, A.T.; Loiseau, P.M.; Figadère, B. Synthesis and Antiprotozoal Activity of Original Porphyrin Precursors and Derivatives. Eur. J. Med. Chem. 2013, 67, 158–165.
- Espitia-Almeida, F.; Díaz-Uribe, C.; Vallejo, W.; Gómez-Camargo, D.; Romero Bohórquez, A.R. In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania Braziliensis and Leishmania Panamensis Promastigotes Properties. Molecules 2020, 25, 1887.
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation beyond the Medical Scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57.
- de Souza, L.M.; Venturini, F.P.; Inada, N.M.; Iermak, I.; Garbuio, M.; Mezzacappo, N.F.; de Oliveira, K.T.; Bagnato, V.S. Curcumin in Formulations against Aedes Aegypti: Mode of Action, Photolarvicidal and Ovicidal Activity. Photodiagn. Photodyn. Ther. 2020, 31, 101840.
- Khater, H.; Hendawy, N.; Govindarajan, M.; Murugan, K.; Benelli, G. Photosensitizers in the Fight against Ticks: Safranin as a Novel Photodynamic Fluorescent Acaricide to Control the Camel Tick Hyalomma Dromedarii (Ixodidae). Parasitol. Res. 2016, 115, 3747–3758.
- Karolina Wojewoda; Martin Gillstedt; Jonatan Tovi; Louai Salah; Ann-Marie Wennberg Larkö; Alexandra Sjöholm; Carin Sandberg; Optimizing treatment of acne with photodynamic therapy (PDT) to achieve long-term remission and reduce side effects. A prospective randomized controlled trial. Journal of Photochemistry and Photobiology B: Biology 2021, 223, 112299, 10.1016/j.jphotobiol.2021.112299.
- Mannucci, E.; Genovese, S.; Monami, M.; Navalesi, G.; Dotta, F.; Anichini, R.; Romagnoli, F.; Gensini, G. Photodynamic Topical Antimicrobial Therapy for Infected Foot Ulcers in Patients with Diabetes: A Randomized, Double-Blind, Placebo-Controlled Study—The D.A.N.T.E (Diabetic Ulcer Antimicrobial New Topical Treatment Evaluation) Study. Acta Diabetol. 2014, 51, 435–440.
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.L.; Faris, B.; Young, R.J.; Brown, S.B.; et al. Phase IIa Randomized, Placebo-Controlled Study of Antimicrobial Photodynamic Therapy in Bacterially Colonized, Chronic Leg Ulcers and Diabetic Foot Ulcers: A New Approach to Antimicrobial Therapy. Br. J. Dermatol. 2013, 168, 617–624.
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic Therapy of Interdigital Mycoses of the Feet with Topical Application of 5-Aminolevulinic Acid. Photodermatol. Photoimmunol. Photomed. 2004, 20, 144–147.
- Lin, J.; Wan, M.T. Current Evidence and Applications of Photodynamic Therapy in Dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145.
- Tardivo, J.P.; Adami, F.; Correa, J.A.; Pinhal, M.A.S.; Baptista, M.S. A Clinical Trial Testing the Efficacy of PDT in Preventing Amputation in Diabetic Patients. Photodiagn. Photodyn. Ther. 2014, 11, 342–350.
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter Pylori Accumulates Photoactive Porphyrins and Is Killed by Visible Light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827.
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; Baptista, M.d.S.; Lincopan, N. Global Priority Multidrug-Resistant Pathogens Do Not Resist Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111893.
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure-Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239.
- Marasini, S.; Leanse, L.G.; Dai, T. Can Microorganisms Develop Resistance against Light Based Anti-Infective Agents? Adv. Drug Deliv. Rev. 2021, 175, 113822.
- Tschowri, N.; Lindenberg, S.; Hengge, R. Molecular Function and Potential Evolution of the Biofilm-Modulating Blue Light-Signalling Pathway of Escherichia Coli. Mol. Microbiol. 2012, 85, 893–906.
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of Multidrug Efflux Systems on Methylene Blue-Mediated Photodynamic Inactivation of Candida Albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532.
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of Bacterial Multidrug Efflux Pumps Potentiate Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209.
- Khan, S.; Khan, S.N.; Akhtar, F.; Misba, L.; Meena, R.; Khan, A.U. Inhibition of Multi-Drug Resistant Klebsiella Pneumoniae: Nanoparticles Induced Photoinactivation in Presence of Efflux Pump Inhibitor. Eur. J. Pharm. Biopharm. 2020, 157, 165–174.
- Kashef, N.; Akbarizare, M.; Kamrava, S.K. Effect of Sub-Lethal Photodynamic Inactivation on the Antibiotic Susceptibility and Biofilm Formation of Clinical Staphylococcus Aureus Isolates. Photodiagn. Photodyn. Ther. 2013, 10, 368–373.
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299.
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107.
- Wozniak, A.; Grinholc, M. Combined Antimicrobial Activity of Photodynamic Inactivation and Antimicrobials—State of the Art. Front. Microbiol. 2018, 9, 930.
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364.
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302.
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584.
- Gollmer, A.; Felgenträger, A.; Bäumler, W.; Maisch, T.; Späth, A. A Novel Set of Symmetric Methylene Blue Derivatives Exhibits Effective Bacteria Photokilling—A Structure–Response Study. Photochem. Photobiol. Sci. 2015, 14, 335–351.
- Ahmad, A. Phthalocyanines Derivatives as Control Approach for Antimicrobial Photodynamic Therapy. J. Am. J. Clin. Microbiol. Antimicrob. 2019, 2, 8.
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456.
- Jeon, Y.-M.; Lee, H.-S.; Jeong, D.; Oh, H.-K.; Ra, K.-H.; Lee, M.-Y. Antimicrobial Photodynamic Therapy Using Chlorin E6 with Halogen Light for Acne Bacteria-Induced Inflammation. Life Sci. 2015, 124, 56–63.
- Li, X.; Lee, D.; Huang, J.-D.; Yoon, J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 9885–9890.
- Terra Garcia, M.; Correia Pereira, A.H.; Figueiredo-Godoi, L.M.A.; Jorge, A.O.C.; Strixino, J.F.; Junqueira, J.C. Photodynamic Therapy Mediated by Chlorin-Type Photosensitizers against Streptococcus Mutans Biofilms. Photodiagn. Photodyn. Ther. 2018, 24, 256–261.
- Zhang, Y.; Lovell, J.F. Recent Applications of Phthalocyanines and Naphthalocyanines for Imaging and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1420.
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch Oral Biol. 2021, 122, 105024.
- Galdino, D.Y.T.; da Rocha Leódido, G.; Pavani, C.; Gonçalves, L.M.; Bussadori, S.K.; Benini Paschoal, M.A. Photodynamic Optimization by Combination of Xanthene Dyes on Different Forms of Streptococcus Mutans: An In Vitro Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102191.
- Silva, A.F.; Dos Santos, A.R.; Trevisan, D.A.C.; Bonin, E.; Freitas, C.F.; Batista, A.F.P.; Hioka, N.; Simões, M.; Graton Mikcha, J.M. Xanthene Dyes and Green LED for the Inactivation of Foodborne Pathogens in Planktonic and Biofilm States. Photochem Photobiol 2019, 95, 1230–1238.
- Mizuno, K.; Zhiyentayev, T.; Huang, L.; Khalil, S.; Nasim, F.; Tegos, G.P.; Gali, H.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-Activity Relationships. J. Nanomed. Nanotechnol. 2011, 2, 1–9.
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes In Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825.
- Yin, R.; Hamblin, M. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185.
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.-T.; Yu, R.-S.; Hsieh, M.F.; Chen, J.-C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598.
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met.-Based Drugs 2008, 2008, 1–23.
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90.
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639.
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding Medicinal Chemistry into 3D Space: Metallofragments as 3D Scaffolds for Fragment-Based Drug Discovery. Chem. Sci. 2020, 11, 1216–1225.
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.R. Diversity-Oriented Synthesis as a Tool for the Discovery of Novel Biologically Active Small Molecules. Nat. Commun. 2010, 1, 80.
- Hung, A.W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J.A.; Clemons, P.A.; Young, D.W. Route to Three-Dimensional Fragments Using Diversity-Oriented Synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804.
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 56.
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542.
- Deng, T.; Zhao, H.; Shi, M.; Qiu, Y.; Jiang, S.; Yang, X.; Zhao, Y.; Zhang, Y. Photoactivated Trifunctional Platinum Nanobiotics for Precise Synergism of Multiple Antibacterial Modes. Small 2019, 15, 1902647.
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic Octahedral Molybdenum Cluster Complexes Functionalized with Mitochondria-Targeting Ligands: Photodynamic Anticancer and Antibacterial Activities. Biomater. Sci. 2019, 7, 1386–1392.
- Sudhamani, C.N.; Bhojya Naik, H.S.; Sangeetha Gowda, K.R.; Girija, D.; Giridhar, M. DNA Binding, Prominent Photonuclease Activity and Antibacterial PDT of Cobalt(II) Complexes of Phenanthroline Based Photosensitizers. Nucleosides Nucleotides Nucleic Acids 2018, 37, 546–562.
- Wang, L.; Monro, S.; Cui, P.; Yin, H.; Liu, B.; Cameron, C.G.; Xu, W.; Hetu, M.; Fuller, A.; Kilina, S.; et al. Heteroleptic Ir(III)N 6 Complexes with Long-Lived Triplet Excited States and In Vitro Photobiological Activities. ACS Appl. Mater. Interfaces 2019, 11, 3629–3644.
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874.
- Jain, A.; Garrett, N.T.; Malone, Z.P. Ruthenium-based Photoactive Metalloantibiotics. Photochem. Photobiol. 2021, php.13435.
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2014, 57, 7280–7292.
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450.
- Sun, W.; Jian, Y.; Zhou, M.; Yao, Y.; Tian, N.; Li, C.; Chen, J.; Wang, X.; Zhou, Q. Selective and Efficient Photoinactivation of Intracellular Staphylococcus Aureus and MRSA with Little Accumulation of Drug Resistance: Application of a Ru(II) Complex with Photolabile Ligands. J. Med. Chem. 2021, 64, 7359–7370.
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 Spike-Host Cell Receptor GRP78 Binding Site Prediction. J. Infect. 2020, 80, 554–562.
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249.
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142.
- Khorsandi, K.; Fekrazad, S.; Vahdatinia, F.; Farmany, A.; Fekrazad, R. Nano Antiviral Photodynamic Therapy: A Probable Biophysicochemical Management Modality in SARS-CoV-2. Expert Opin. Drug Deliv. 2021, 18, 265–272.
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424.
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their Potential Use in Antibacterial Photodynamic Therapy. Photochem. Photobiol. Sci. 2011, 10, 712–720.
- Basavegowda, N.; Baek, K.-H. Multimetallic Nanoparticles as Alternative Antimicrobial Agents: Challenges and Perspectives. Molecules 2021, 26, 912.
- Zhou, Z.; Peng, S.; Sui, M.; Chen, S.; Huang, L.; Xu, H.; Jiang, T. Multifunctional Nanocomplex for Surface-Enhanced Raman Scattering Imaging and near-Infrared Photodynamic Antimicrobial Therapy of Vancomycin-Resistant Bacteria. Colloids Surf. B Biointerfaces 2018, 161, 394–402.
- Gupta, A.; Landis, R.F.; Rotello, V.M. Nanoparticle-Based Antimicrobials: Surface Functionality Is Critical. F1000Res 2016, 5.
- Alfirdous, R.A.; Garcia, I.M.; Balhaddad, A.A.; Collares, F.M.; Martinho, F.C.; Melo, M.A.S. Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches. Appl. Sci. 2021, 11, 4759.
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130.
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193.
- Nair, A.B.; Morsy, M.A.; Shinu, P.; Kotta, S.; Chandrasekaran, M.; Tahir, A. Advances of Non-iron Metal Nanoparticles in Biomedicine. J. Pharm. Pharm. Sci. 2021, 24, 41–61.
- Belekov, E.; Kholikov, K.; Cooper, L.; Banga, S.; Er, A.O. Improved Antimicrobial Properties of Methylene Blue Attached to Silver Nanoparticles. Photodiagn. Photodyn. Ther. 2020, 32, 102012.
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874.
- Mottais, A.; Berchel, M.; Sibiril, Y.; Laurent, V.; Gill, D.; Hyde, S.; Jaffrès, P.-A.; Montier, T.; Le Gall, T. Antibacterial Effect and DNA Delivery Using a Combination of an Arsonium-Containing Lipophosphoramide with an N-Heterocyclic Carbene-Silver Complex—Potential Benefits for Cystic Fibrosis Lung Gene Therapy. Int. J. Pharm. 2018, 536, 29–41.
- Ribeiro, M.S.; de Melo, L.S.A.; Farooq, S.; Baptista, A.; Kato, I.T.; Núñez, S.C.; de Araujo, R.E. Photodynamic Inactivation Assisted by Localized Surface Plasmon Resonance of Silver Nanoparticles: In Vitro Evaluation on Escherichia Coli and Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2018, 22, 191–196.
- Yamamoto, M.; Shitomi, K.; Miyata, S.; Miyaji, H.; Aota, H.; Kawasaki, H. Bovine Serum Albumin-Capped Gold Nanoclusters Conjugating with Methylene Blue for Efficient 1O2 Generation via Energy Transfer. J. Colloid Interface Sci. 2018, 510, 221–227.
- Rizzi, V.; Vurro, D.; Placido, T.; Fini, P.; Petrella, A.; Semeraro, P.; Cosma, P. Gold-Chlorophyll a-Hybrid Nanoparticles and Chlorophyll a/Cetyltrimethylammonium Chloride Self-Assembled-Suprastructures as Novel Carriers for Chlorophyll a Delivery in Water Medium: Photoactivity and Photostability. Colloids Surf. B Biointerfaces 2018, 161, 555–562.
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial Photodynamic Activity and Cytocompatibility of Au25(Capt)18 Clusters Photoexcited by Blue LED Light Irradiation. Int. J. Nanomed. 2017, 12, 2703–2716.
- Kubheka, G.; Uddin, I.; Amuhaya, E.; Mack, J.; Nyokong, T. Synthesis and Photophysicochemical Properties of BODIPY Dye Functionalized Gold Nanorods for Use in Antimicrobial Photodynamic Therapy. J. Porphyr. Phthalocyanines 2016, 20, 1016–1024.
- Okamoto, I.; Miyaji, H.; Miyata, S.; Shitomi, K.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Enya, S.; Saita, S.; Kawasaki, H. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega 2021, 6, 9279–9290.
- Rigotto Caruso, G.; Tonani, L.; Marcato, P.D.; von Zeska Kress, M.R. Phenothiazinium Photosensitizers Associated with Silver Nanoparticles in Enhancement of Antimicrobial Photodynamic Therapy. Antibiotics 2021, 10, 569.
- Morales-de-Echegaray, A.V.; Lin, L.; Sivasubramaniam, B.; Yermembetova, A.; Wang, Q.; Abutaleb, N.S.; Seleem, M.N.; Wei, A. Antimicrobial Photodynamic Activity of Gallium-Substituted Haemoglobin on Silver Nanoparticles. Nanoscale 2020, 12, 21734–21742.
- Parasuraman, P.; Thamanna, R.Y.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Pharmaceutics 2020, 12, 709.
- Chen, J.; Yang, L.; Chen, J.; Liu, W.; Zhang, D.; Xu, P.; Dai, T.; Shang, L.; Yang, Y.; Tang, S.; et al. Composite of Silver Nanoparticles and Photosensitizer Leads to Mutual Enhancement of Antimicrobial Efficacy and Promotes Wound Healing. Chem. Eng. J. 2019, 374, 1373–1381.
- Sen, P.; Nyokong, T. Enhanced Photodynamic Inactivation of Staphylococcus Aureus with Schiff Base Substituted Zinc Phthalocyanines through Conjugation to Silver Nanoparticles. J. Mol. Struct. 2021, 1232, 130012.
- Shabangu, S.M.; Babu, B.; Soy, R.C.; Managa, M.; Sekhosana, K.E.; Nyokong, T. Photodynamic Antimicrobial Chemotherapy of Asymmetric Porphyrin-Silver Conjugates towards Photoinactivation of Staphylococcus Aureus. J. Coord. Chem. 2020, 73, 593–608.
- Wanarska, E.; Maliszewska, I. Gold Nanoparticles in an Enhancement of Antimicrobial Activity. Physicochem. Probl. Miner. Process. 2020, 56, 269–279.
- Managa, M.; Antunes, E.; Nyokong, T. Conjugates of Platinum Nanoparticles with Gallium Tetra—(4-Carboxyphenyl) Porphyrin and Their Use in Photodynamic Antimicrobial Chemotherapy When in Solution or Embedded in Electrospun Fiber. Polyhedron 2014, 76, 94–101.
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029.
- Zhen, X.; Chudal, L.; Pandey, N.K.; Phan, J.; Ran, X.; Amador, E.; Huang, X.; Johnson, O.; Ran, Y.; Chen, W.; et al. A Powerful Combination of Copper-Cysteamine Nanoparticles with Potassium Iodide for Bacterial Destruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110659.
- Sah, B.; Wu, J.; Vanasse, A.; Pandey, N.K.; Chudal, L.; Huang, Z.; Song, W.; Yu, H.; Ma, L.; Chen, W.; et al. Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-Ray Induced Photodynamic Therapy. Nanomaterials 2020, 10, 1087.
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545.
- Namanga, J.; Foba, J.; Ndinteh, D.T.; Yufanyi, D.M.; Krause, R.W.M. Synthesis and Magnetic Properties of a Superparamagnetic Nanocomposite "Pectin-Magnetite Nanocomposite". J. Nanomater. 2013, 2013, 87.
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-Isolation of Superparamagnetic Iron Oxide Nanoparticles Improves MRI, MPI and Hyperthermia Performance. J. Nanobiotechnol. 2020, 18, 22.
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A New Insight on Nanomagnet–Porphyrin Hybrids for Photodynamic Inactivation of Microorganisms. Dye. Pigment. 2014, 110, 80–88.
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F.; Yagci Acar, H. Broad Spectrum Antibacterial Photodynamic and Photothermal Therapy Achieved with Indocyanine Green Loaded SPIONs under near Infrared Irradiation. Biomater. Sci. 2020, 8, 4616–4625.
- de Santana, W.M.O.S.; Caetano, B.L.; de Annunzio, S.R.; Pulcinelli, S.H.; Ménager, C.; Fontana, C.R.; Santilli, C.V. Conjugation of Superparamagnetic Iron Oxide Nanoparticles and Curcumin Photosensitizer to Assist in Photodynamic Therapy. Colloids Surf. B Biointerfaces 2020, 196, 111297.
- Dube, E.; Soy, R.; Shumba, M.; Nyokong, T. Photophysicochemical Behaviour of Phenoxy Propanoic Acid Functionalised Zinc Phthalocyanines When Grafted onto Iron Oxide and Silica Nanoparticles: Effects in Photodynamic Antimicrobial Chemotherapy. J. Lumin. 2021, 234, 117939.
- Mapukata, S.; Nwahara, N.; Nyokong, T. The Photodynamic Antimicrobial Chemotherapy of Stapphylococcus Aureus Using an Asymmetrical Zinc Phthalocyanine Conjugated to Silver and Iron Oxide Based Nanoparticles. J. Photochem. Photobiol. A Chem. 2020, 402, 112813.
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Magnetic Nanoplatforms for In Situ Modification of Macromolecules: Synthesis, Characterization, and Photoinactivating Power of Cationic Nanoiman–Porphyrin Conjugates. ACS Appl. Bio Mater. 2020, 3, 5930–5940.
- Scanone, A.C.; Santamarina, S.C.; Heredia, D.A.; Durantini, E.N.; Durantini, A.M. Functionalized Magnetic Nanoparticles with BODIPYs for Bioimaging and Antimicrobial Therapy Applications. ACS Appl. Bio Mater. 2020, 3, 1061–1070.
- Sun, X.; Wang, L.; Lynch, C.D.; Sun, X.; Li, X.; Qi, M.; Ma, C.; Li, C.; Dong, B.; Zhou, Y.; et al. Nanoparticles Having Amphiphilic Silane Containing Chlorin E6 with Strong Anti-Biofilm Activity against Periodontitis-Related Pathogens. J. Dent. 2019, 81, 70–84.
- Toledo, V.H.; Yoshimura, T.M.; Pereira, S.T.; Castro, C.E.; Ferreira, F.F.; Ribeiro, M.S.; Haddad, P.S. Methylene Blue-Covered Superparamagnetic Iron Oxide Nanoparticles Combined with Red Light as a Novel Platform to Fight Non-Local Bacterial Infections: A Proof of Concept Study against Escherichia Coli. J. Photochem. Photobiol. B 2020, 209, 111956.
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel Nanomaterial-Based Antibacterial Photodynamic Therapies to Combat Oral Bacterial Biofilms and Infectious Diseases. Int. J. Nanomed. 2019, 14, 6937–6956.
- Collen Makola, L.; Nyokong, T.; Amuhaya, E.K. Impact of Axial Ligation on Photophysical and Photodynamic Antimicrobial Properties of Indium (III) Methylsulfanylphenyl Porphyrin Complexes Linked to Silver-Capped Copper Ferrite Magnetic Nanoparticles. Polyhedron 2021, 193, 114882.
- Makola, L.C.; Managa, M.; Nyokong, T. Enhancement of Photodynamic Antimicrobialtherapy through the Use of Cationic Indium Porphyrin Conjugated to Ag/CuFe2O4 Nanoparticles. Photodiagn. Photodyn. Ther. 2020, 30, 101736.
- Philip, S.; Kuriakose, S. Photodynamic Antifungal Activity of a Superparamagnetic and Fluorescent Drug Carrier System against Antibiotic-Resistant Fungal Strains. Cellulose 2021, 28, 9091–9102.
- Sun, X.; Sun, J.; Sun, Y.; Li, C.; Fang, J.; Zhang, T.; Wan, Y.; Xu, L.; Zhou, Y.; Wang, L.; et al. Oxygen Self-Sufficient Nanoplatform for Enhanced and Selective Antibacterial Photodynamic Therapy against Anaerobe-Induced Periodontal Disease. Adv. Funct. Mater. 2021, 31, 2101040.
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141.
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481.
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410.
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387.
- Joe, A.; Park, S.-H.; Shim, K.-D.; Kim, D.-J.; Jhee, K.-H.; Lee, H.-W.; Heo, C.-H.; Kim, H.-M.; Jang, E.-S. Antibacterial Mechanism of ZnO Nanoparticles under Dark Conditions. J. Ind. Eng. Chem. 2017, 45, 430–439.
- Liou, J.-W.; Chang, H.-H. Bactericidal Effects and Mechanisms of Visible Light-Responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275.
- Oves, M.; Arshad, M.; Khan, M.S.; Ahmed, A.S.; Azam, A.; Ismail, I.M.I. Anti-Microbial Activity of Cobalt Doped Zinc Oxide Nanoparticles: Targeting Water Borne Bacteria. J. Saudi Chem. Soc. 2015, 19, 581–588.
- Raut, A.V.; Yadav, H.M.; Gnanamani, A.; Pushpavanam, S.; Pawar, S.H. Synthesis and Characterization of Chitosan-TiO2:Cu Nanocomposite and Their Enhanced Antimicrobial Activity with Visible Light. Colloids Surf. B Biointerfaces 2016, 148, 566–575.
- Xu, X.; Dutta, A.; Khurgin, J.; Wei, A.; Shalaev, V.M.; Boltasseva, A. 2 Core–Shell Nanoparticles as Plasmon-Enhanced Photosensitizers: The Role of Hot Electron Injection. Laser Photonics Rev. 2020, 14, 1900376.
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial Surfaces and Their Potential in Reducing the Role of the Inanimate Environment in the Incidence of Hospital-Acquired Infections. J. Mater. Chem. 2009, 19, 3819–3831.
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and Antimicrobial Activities of Chitosan-TiO2 Nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773.
- Pantaroto, H.N.; Ricomini-Filho, A.P.; Bertolini, M.M.; Dias da Silva, J.H.; Azevedo Neto, N.F.; Sukotjo, C.; Rangel, E.C.; Barão, V.A.R. Antibacterial Photocatalytic Activity of Different Crystalline TiO2 Phases in Oral Multispecies Biofilm. Dent. Mater. 2018, 34, e182–e195.
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An Antibacterial Surface on Dental Implants, Based on the Photocatalytic Bactericidal Effect. Clin. Implant. Dent. Relat. Res. 2005, 7, 105–111.
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241.
- De Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles; IntechOpen: London, UK, 2020; ISBN 978-1-83962-433-9.
- Farkhonde Masoule, S.; Pourhajibagher, M.; Safari, J.; Khoobi, M. Base-Free Green Synthesis of Copper(II) Oxide Nanoparticles Using Highly Cross-Linked Poly(Curcumin) Nanospheres: Synergistically Improved Antimicrobial Activity. Res. Chem. Intermed. 2019, 45, 4449–4462.
- Varaprasad, K.; López, M.; Núñez, D.; Jayaramudu, T.; Sadiku, E.R.; Karthikeyan, C.; Oyarzúnc, P. Antibiotic Copper Oxide-Curcumin Nanomaterials for Antibacterial Applications. J. Mol. Liq. 2020, 300, 112353.
- Xiu, W.; Gan, S.; Wen, Q.; Qiu, Q.; Dai, S.; Dong, H.; Li, Q.; Yuwen, L.; Weng, L.; Teng, Z.; et al. Biofilm Microenvironment-Responsive Nanotheranostics for Dual-Mode Imaging and Hypoxia-Relief-Enhanced Photodynamic Therapy of Bacterial Infections. Research 2020, 2020, 9426453.
- Sambhaji, R.B.; Vijay, J.S. Folate Tethered Gd2O3 Nanoparticles Exhibit Photoactive Antimicrobial Effects and pH Responsive Delivery of 5-Fluorouracil into MCF-7 Cells. Drug Deliv. Lett. 2019, 9, 58–68.
- Hossain, M.K.; Khan, M.I.; El-Denglawey, A. A Review on Biomedical Applications, Prospects, and Challenges of Rare Earth Oxides. Appl. Mater. Today 2021, 24, 101104.
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431.
- Tang, Y.; Qin, Z.; Yin, S.; Sun, H. Transition Metal Oxide and Chalcogenide-Based Nanomaterials for Antibacterial Activities: An Overview. Nanoscale 2021, 13, 6373–6388.
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670.
- McCollum, C.R.; Levy, M.; Bertram, J.R.; Nagpal, P.; Chatterjee, A. Photoexcited Quantum Dots as Efficacious and Nontoxic Antibiotics in an Animal Model. ACS Biomater. Sci. Eng. 2021, 7, 1863–1875.
- Nain, A.; Wei, S.-C.; Lin, Y.-F.; Tseng, Y.-T.; Mandal, R.P.; Huang, Y.-F.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Copper Sulfide Nanoassemblies for Catalytic and Photoresponsive Eradication of Bacteria from Infected Wounds. ACS Appl. Mater. Interfaces 2021, 13, 7865–7878.
- Jiang, C.; Scholle, F.; Ghiladi, R.A.; Dai, T.; Wu, M.X.; Popp, J. Mn-Doped Zn/S Quantum Dots as Photosensitizers for Antimicrobial Photodynamic Inactivation. In Photonic Diagnosis and Treatment of Infections and Inflammatory Diseases II; SPIE BiOS: San Francisco, CA, USA, 2019; p. 10863.
- McCollum, C.R.; Bertram, J.R.; Nagpal, P.; Chatterjee, A. Photoactivated Indium Phosphide Quantum Dots Treat Multidrug-Resistant Bacterial Abscesses In Vivo. ACS Appl. Mater. Interfaces 2021, 13, 30404–30419.
- Tian, X.; Sun, Y.; Fan, S.; Boudreau, M.D.; Chen, C.; Ge, C.; Yin, J.-J. Photogenerated Charge Carriers in Molybdenum Disulfide Quantum Dots with Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2019, 11, 4858–4866.
- Narband, N.; Mubarak, M.; Ready, D.; Parkin, I.P.; Nair, S.P.; Green, M.A.; Beeby, A.; Wilson, M. Quantum Dots as Enhancers of the Efficacy of Bacterial Lethal Photosensitization. Nanotechnology 2008, 19, 445102.
- Nadeau, J.; Chibli, H.; Carlini, L. Photosensitization of InP/ZnS Quantum Dots for Anti-Cancer and Anti-Microbial Applications. In Proceedings of the Colloidal Nanocrystals for Biomedical Applications VII; SPIE: San Francisco, CA, USA, 2012; Volume 8232, pp. 87–95.
- Liu, J.; Cheng, W.; Wang, Y.; Fan, X.; Shen, J.; Liu, H.; Wang, A.; Hui, A.; Nichols, F.; Chen, S. Cobalt-Doped Zinc Oxide Nanoparticle–MoS2 Nanosheet Composites as Broad-Spectrum Bactericidal Agents. ACS Appl. Nano Mater. 2021, 4, 4361–4370.
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nat. Mater. 2016, 15, 529–534.
- Goodman, S.M.; Levy, M.; Li, F.-F.; Ding, Y.; Courtney, C.M.; Chowdhury, P.P.; Erbse, A.; Chatterjee, A.; Nagpal, P. Designing Superoxide-Generating Quantum Dots for Selective Light-Activated Nanotherapy. Front. Chem. 2018, 6, 46.
- Ganguly, P.; Mathew, S.; Clarizia, L.; Kumar, R.S.; Akande, A.; Hinder, S.; Breen, A.; Pillai, S.C. Theoretical and Experimental Investigation of Visible Light Responsive AgBiS2-TiO2 Heterojunctions for Enhanced Photocatalytic Applications. Appl. Catal. B Environ. 2019, 253, 401–418.
- Nazir, M.; Aziz, M.I.; Ali, I.; Basit, M.A. Revealing Antimicrobial and Contrasting Photocatalytic Behavior of Metal Chalcogenide Deposited P25-TiO2 Nanoparticles. Photonics Nanostruct.-Fundam. Appl. 2019, 36, 100721.
- Suganya, M.; Balu, A.R.; Balamurugan, S.; Srivind, J.; Narasimman, V.; Manjula, N.; Rajashree, C.; Nagarethinam, V.S. Photoconductive, Photocatalytic and Antifungal Properties of PbS:Mo Nanoparticles Synthesized via Precipitation Method. Surf. Interfaces 2018, 13, 148–156.
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672.
- Qian, S.; Song, L.; Sun, L.; Zhang, X.; Xin, Z.; Yin, J.; Luan, S. Metal-Organic Framework/Poly (ε-Caprolactone) Hybrid Electrospun Nanofibrous Membranes with Effective Photodynamic Antibacterial Activities. J. Photochem. Photobiol. A Chem. 2020, 400, 112626.
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Wei, Q. FRET as a Novel Strategy to Enhance the Singlet Oxygen Generation of Porphyrinic MOF Decorated Self-Disinfecting Fabrics. Chem. Eng. J. 2020, 395, 125012.
- Yu, P.; Han, Y.; Han, D.; Liu, X.; Liang, Y.; Li, Z.; Zhu, S.; Wu, S. In-Situ Sulfuration of Cu-Based Metal-Organic Framework for Rapid near-Infrared Light Sterilization. J. Hazard. Mater. 2020, 390, 122126.
- Xiong, K.; Li, J.; Tan, L.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Liu, X. Ag2S Decorated Nanocubes with Enhanced Near-Infrared Photothermal and Photodynamic Properties for Rapid Sterilization. Colloid Interface Sci. Commun. 2019, 33, 100201.
- Bagchi, D.; Bhattacharya, A.; Dutta, T.; Nag, S.; Wulferding, D.; Lemmens, P.; Pal, S.K. Nano MOF Entrapping Hydrophobic Photosensitizer for Dual-Stimuli-Responsive Unprecedented Therapeutic Action against Drug-Resistant Bacteria. ACS Appl. Bio Mater. 2019, 2, 1772–1780.
- Golmohamadpour, A.; Bahramian, B.; Khoobi, M.; Pourhajibagher, M.; Barikani, H.R.; Bahador, A. Antimicrobial Photodynamic Therapy Assessment of Three Indocyanine Green-Loaded Metal-Organic Frameworks against Enterococcus Faecalis. Photodiagn. Photodyn. Ther. 2018, 23, 331–338.
- Wang, M.; Nian, L.; Cheng, Y.; Yuan, B.; Cheng, S.; Cao, C. Encapsulation of Colloidal Semiconductor Quantum Dots into Metal-Organic Frameworks for Enhanced Antibacterial Activity through Interfacial Electron Transfer. Chem. Eng. J. 2021, 426, 130832.
- Hynek, J.; Zelenka, J.; Rathouský, J.; Kubát, P.; Ruml, T.; Demel, J.; Lang, K. Designing Porphyrinic Covalent Organic Frameworks for the Photodynamic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8527–8535.
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent Progress in the Development of Upconversion Nanomaterials in Bioimaging and Disease Treatment. J. Nanobiotechnol. 2020, 18, 154.
- Zhang, T.; Ying, D.; Qi, M.; Li, X.; Fu, L.; Sun, X.; Wang, L.; Zhou, Y. Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin E6 against Periodontal Pathogens. Molecules 2019, 24, 2692.
- Chen, B.; Wang, F. Emerging Frontiers of Upconversion Nanoparticles. Trends Chem. 2020, 2, 427–439.
- Yao, J.; Huang, C.; Liu, C.; Yang, M. Upconversion Luminescence Nanomaterials: A Versatile Platform for Imaging, Sensing, and Therapy. Talanta 2020, 208, 120157.
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic Lysozyme-Photodynamic Therapy Against Resistant Bacteria Based on an Intelligent Upconversion Nanoplatform. Angew. Chem. Int. Ed. 2021, 60, 19201–19206.
- Wang, Z.; Liu, C.; Zhao, Y.; Hu, M.; Ma, D.; Zhang, P.; Xue, Y.; Li, X. Photomagnetic Nanoparticles in Dual-Modality Imaging and Photo-Sonodynamic Activity against Bacteria. Chem. Eng. J. 2019, 356, 811–818.
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual Antibacterial Activities of a Chitosan-Modified Upconversion Photodynamic Therapy System against Drug-Resistant Bacteria in Deep Tissue. Nanoscale 2017, 9, 3912–3924.
- Zhang, Y.; Huang, P.; Wang, D.; Chen, J.; Liu, W.; Hu, P.; Huang, M.; Chen, X.; Chen, Z. Near-Infrared-Triggered Antibacterial and Antifungal Photodynamic Therapy Based on Lanthanide-Doped Upconversion Nanoparticles. Nanoscale 2018, 10, 15485–15495.
- Sharma, B.; Kaur, G.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P.A. High Antimicrobial Photodynamic Activity of Photosensitizer Encapsulated Dual-Functional Metallocatanionic Vesicles against Drug-Resistant Bacteria S. Aureus. Biomater. Sci. 2020, 8, 2905–2920.
- Kaur, G.; Berwal, K.; Sharma, B.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P.A. Enhanced Antimicrobial Photodynamic Activity of Photosensitizer Encapsulated Copper Based Metallocatanionic Vesicles against E. coli Using Visible Light. J. Mol. Liq. 2021, 324, 114688.
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803.
- Park, Y.; Yoo, J.; Kang, M.-H.; Kwon, W.; Joo, J. Photoluminescent and Biodegradable Porous Silicon Nanoparticles for Biomedical Imaging. J. Mater. Chem. B 2019, 7, 6271–6292.
- Jabir, M.S.; Nayef, U.M.; Jawad, K.H.; Taqi, Z.J.; Buthenhia, A.H.; Ahmed, N.R. Porous Silicon Nanoparticles Prepared via an Improved Method: A Developing Strategy for a Successful Antimicrobial Agent against Escherichia Coli and Staphylococcus Aureus. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012077.
- Xiao, L.; Gu, L.; Howell, S.B.; Sailor, M.J. Porous Silicon Nanoparticle Photosensitizers for Singlet Oxygen and Their Phototoxicity against Cancer Cells. ACS Nano 2011, 5, 3651–3659.
- Secret, E.; Maynadier, M.; Gallud, A.; Gary-Bobo, M.; Chaix, A.; Belamie, E.; Maillard, P.; Sailor, M.J.; Garcia, M.; Durand, J.-O.; et al. Anionic Porphyrin-Grafted Porous Silicon Nanoparticles for Photodynamic Therapy. Chem. Commun. 2013, 49, 4202–4204.
- Chaix, A.; Cheikh, K.E.; Bouffard, E.; Maynadier, M.; Aggad, D.; Stojanovic, V.; Knezevic, N.; Garcia, M.; Maillard, P.; Morère, A.; et al. Mesoporous Silicon Nanoparticles for Targeted Two-Photon Theranostics of Prostate Cancer. J. Mater. Chem. B 2016, 4, 3639–3642.
- Näkki, S.; Martinez, J.O.; Evangelopoulos, M.; Xu, W.; Lehto, V.-P.; Tasciotti, E. Chlorin E6 Functionalized Theranostic Multistage Nanovectors Transported by Stem Cells for Effective Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 23441–23449.
- Knežević, N.Ž.; Stojanovic, V.; Chaix, A.; Bouffard, E.; Cheikh, K.E.; Morère, A.; Maynadier, M.; Lemercier, G.; Garcia, M.; Gary-Bobo, M.; et al. Ruthenium(II) Complex-Photosensitized Multifunctionalized Porous Silicon Nanoparticles for Two-Photon near-Infrared Light Responsive Imaging and Photodynamic Cancer Therapy. J. Mater. Chem. B 2016, 4, 1337–1342.
- Capeletti, L.B.; de Oliveira, L.F.; Gonçalves, K.d.A.; de Oliveira, J.F.A.; Saito, Â.; Kobarg, J.; dos Santos, J.H.Z.; Cardoso, M.B. Tailored Silica–Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity. Langmuir 2014, 30, 7456–7464.
- Guo, Y.; Rogelj, S.; Zhang, P. Rose Bengal-Decorated Silica Nanoparticles as Photosensitizers for Inactivation of Gram-Positive Bacteria. Nanotechnology 2010, 21, 065102.
- Wang, C.; Chen, P.; Qiao, Y.; Kang, Y.; Yan, C.; Yu, Z.; Wang, J.; He, X.; Wu, H. PH Responsive Superporogen Combined with PDT Based on Poly Ce6 Ionic Liquid Grafted on SiO2 for Combating MRSA Biofilm Infection. Theranostics 2020, 10, 4795–4808.
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic Properties and Photoinactivation of Microorganisms Mediated by 5,10,15,20-Tetrakis(4-Carboxyphenyl)Porphyrin Covalently Linked to Silica-Coated Magnetite Nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 346, 452–461.
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-Fused Chlorin Photosensitizer Immobilized on Solid Supports for the Photoinactivation of Gram Negative Bacteria. Dye. Pigment. 2014, 110, 123–133.
- Baigorria, E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Silica Nanoparticles Embedded with Water Insoluble Phthalocyanines for the Photoinactivation of Microorganisms. Photodiagn. Photodyn. Ther. 2018, 23, 261–269.
- Amin, A.; Kaduskar, D.V. Comparative Study on Photodynamic Activation of Ortho-Toluidine Blue and Methylene Blue Loaded Mesoporous Silica Nanoparticles Against Resistant Microorganisms. Recent Pat. Drug Deliv. Formul. 2018, 12, 154–161.
- Paramanantham, P.; Siddhardha, B.; Sb, S.L.; Sharan, A.; Alyousef, A.A.; Dosary, M.S.A.; Arshad, M.; Syed, A. Antimicrobial Photodynamic Therapy on Staphylococcus Aureus and Escherichia Coli Using Malachite Green Encapsulated Mesoporous Silica Nanoparticles: An In Vitro Study. PeerJ 2019, 7, e7454.
- Parasuraman, P.; Antony, A.P.; S.B, S.L.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.S.; Syed, A. Antimicrobial Photodynamic Activity of Toluidine Blue Encapsulated in Mesoporous Silica Nanoparticles against Pseudomonas Aeruginosa and Staphylococcus Aureus. Biofouling 2019, 35, 89–103.
- Wysocka-Król, K.; Olsztyńska-Janus, S.; Plesch, G.; Plecenik, A.; Podbielska, H.; Bauer, J. Nano-Silver Modified Silica Particles in Antibacterial Photodynamic Therapy. Appl. Surf. Sci. 2018, 461, 260–268.
- Liu, Y.; Liu, X.; Xiao, Y.; Chen, F.; Xiao, F. A Multifunctional Nanoplatform Based on Mesoporous Silica Nanoparticles for Imaging-Guided Chemo/Photodynamic Synergetic Therapy. RSC Adv. 2017, 7, 31133–31141.
- Grüner, M.C.; Arai, M.S.; Carreira, M.; Inada, N.; de Camargo, A.S.S. Functionalizing the Mesoporous Silica Shell of Upconversion Nanoparticles to Enhance Bacterial Targeting and Killing via Photosensitizer-Induced Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1028–1036.
- Mapukata, S.; Britton, J.; Osifeko, O.L.; Nyokong, T. The Improved Antibacterial Efficiency of a Zinc Phthalocyanine When Embedded on Silver Nanoparticle Modified Silica Nanofibers. Photodiagn. Photodyn. Ther. 2021, 33, 102100.
- Al-Omari, S. Toward a Molecular Understanding of the Photosensitizer-Copper Interaction for Tumor Destruction. Biophys. Rev. 2013, 5, 305–311.
- Eckl, D.B.; Dengler, L.; Nemmert, M.; Eichner, A.; Bäumler, W.; Huber, H. A Closer Look at Dark Toxicity of the Photosensitizer TMPyP in Bacteria. Photochem. Photobiol. 2018, 94, 165–172.
- Ma, J.; Liu, C.; Yan, K. CQDs-MoS2 QDs Loaded on Dendritic Fibrous Nanosilica/Hydrophobic Waterborne Polyurethane Acrylate for Antibacterial Coatings. Chem. Eng. J. 2022, 429, 132170.
- Zhao, Z.; Yan, R.; Wang, J.; Wu, H.; Wang, Y.; Chen, A.; Shao, S.; Li, Y.-Q. A Bacteria-Activated Photodynamic Nanosystem Based on Polyelectrolyte-Coated Silica Nanoparticles. J. Mater. Chem. B 2017, 5, 3572–3579.
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-Loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284.
- Paramanantham, P.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Applications of Carbon-Based Nanomaterials for Antimicrobial Photodynamic Therapy. In Microbial Nanobionics: Volume 2, Basic Research and Applications; Prasad, R., Ed.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 237–259. ISBN 978-3-030-16534-5.
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized Carbon-Based Nanomaterials and Quantum Dots with Antibacterial Activity: A Review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44.
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196.
- Agazzi, M.L.; Durantini, J.E.; Gsponer, N.S.; Durantini, A.M.; Bertolotti, S.G.; Durantini, E.N. Light-Harvesting Antenna and Proton-Activated Photodynamic Effect of a Novel BODIPY−Fullerene C60 Dyad as Potential Antimicrobial Agent. ChemPhysChem 2019, 20, 1110–1125.
- Agazzi, M.L.; Almodovar, V.A.S.; Gsponer, N.S.; Bertolotti, S.; Tomé, A.C.; Durantini, E.N. Diketopyrrolopyrrole–Fullerene C60 Architectures as Highly Efficient Heavy Atom-Free Photosensitizers: Synthesis, Photophysical Properties and Photodynamic Activity. Org. Biomol. Chem. 2020, 18, 1449–1461.
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A Novel Tricationic Fullerene C60 as Broad-Spectrum Antimicrobial Photosensitizer: Mechanisms of Action and Potentiation with Potassium Iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341.
- Huang, Y.-Y.; Sharma, S.K.; Yin, R.; Agrawal, T.; Chiang, L.Y.; Hamblin, M.R. Functionalized Fullerenes in Photodynamic Therapy. J. Biomed. Nanotechnol. 2014, 10, 1918–1936.
- Palacios, Y.B.; Durantini, J.E.; Heredia, D.A.; Martínez, S.R.; González de la Torre, L.; Durantini, A.M. Tuning the Polarity of Fullerene C60 Derivatives for Enhanced Photodynamic Inactivation. Photochem. Photobiol. 2021.
- Reynoso, E.; Durantini, A.M.; Solis, C.A.; Macor, L.P.; Otero, L.A.; Gervaldo, M.A.; Durantini, E.N.; Heredia, D.A. Photoactive Antimicrobial Coating Based on a PEDOT-Fullerene C60 Polymeric Dyad. RSC Adv. 2021, 11, 23519–23532.
- Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Chapter 18—Fullerene Derivatives in Photodynamic Inactivation of Microorganisms. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–433. ISBN 978-0-323-46152-8.
- Anju, V.T.; Paramanantham, P.; S.B, S.L.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; K., K.; Busi, S. Antimicrobial Photodynamic Activity of Toluidine Blue-Carbon Nanotube Conjugate against Pseudomonas Aeruginosa and Staphylococcus Aureus—Understanding the Mechanism of Action. Photodiagn. Photodyn. Ther. 2019, 27, 305–316.
- Tondro, G.H.; Behzadpour, N.; Keykhaee, Z.; Akbari, N.; Sattarahmady, N. Nanotubes as a Photosensitizer in Laser Phototherapy of Pseudomonas Aeruginosa. Colloids Surf. B Biointerfaces 2019, 180, 481–486.
- Anju, V.T.; Paramanantham, P.; Siddhardha, B.; Lal, S.S.; Sharan, A.; Alyousef, A.A.; Arshad, M.; Syed, A. Malachite Green-Conjugated Multi-Walled Carbon Nanotubes Potentiate Antimicrobial Photodynamic Inactivation of Planktonic Cells and Biofilms of Pseudomonas Aeruginosa and Staphylococcus Aureus. Int. J. Nanomed. 2019, 14, 3861–3874.
- Parasuraman, P.; Anju, V.T.; Lal, S.S.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and Antimicrobial Photodynamic Effect of Methylene Blue Conjugated Carbon Nanotubes on E. Coli and S. Aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576.
- Vt, A.; Paramanantham, P.; Sb, S.L.; Sharan, A.; Alsaedi, M.H.; Dawoud, T.M.S.; Asad, S.; Busi, S. Antimicrobial Photodynamic Activity of Rose Bengal Conjugated Multi Walled Carbon Nanotubes against Planktonic Cells and Biofilm of Escherichia Coli. Photodiagn. Photodyn. Ther. 2018, 24, 300–310.
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial Photodynamic Therapy: Single-Walled Carbon Nanotube (SWCNT)-Porphyrin Conjugate for Visible Light Mediated Inactivation of Staphylococcus Aureus. Colloids Surf. B Biointerfaces 2018, 162, 108–117.
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial Applications of Nanodiamonds. Int. J. Environ. Res Public Health 2016, 13, 413.
- Hurtado, C.R.; Hurtado, G.R.; de Cena, G.L.; Queiroz, R.C.; Silva, A.V.; Diniz, M.F.; dos Santos, V.R.; Trava-Airoldi, V.; Baptista, M.d.S.; Tsolekile, N.; et al. Diamond Nanoparticles-Porphyrin MTHPP Conjugate as Photosensitizing Platform: Cytotoxicity and Antibacterial Activity. Nanomaterials 2021, 11, 1393.
- Openda, Y.I.; Nyokong, T. Enhanced Photo-Ablation Effect of Positively Charged Phthalocyanines-Detonation Nanodiamonds Nanoplatforms for the Suppression of Staphylococcus Aureus and Escherichia Coli Planktonic Cells and Biofilms. J. Photochem. Photobiol. A Chem. 2021, 411, 113200.
- Openda, Y.I.; Nyokong, T. Detonation Nanodiamonds-Phthalocyanine Photosensitizers with Enhanced Photophysicochemical Properties and Effective Photoantibacterial Activity. Photodiagn. Photodyn. Ther. 2020, 32, 102072.
- Openda, Y.I.; Ngoy, B.P.; Nyokong, T. Photodynamic Antimicrobial Action of Asymmetrical Porphyrins Functionalized Silver-Detonation Nanodiamonds Nanoplatforms for the Suppression of Staphylococcus Aureus Planktonic Cells and Biofilms. Front. Chem. 2021, 9, 97.
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon Dots: A Mystic Star in the World of Nanoscience. J. Nanomater. 2019, 2019, e3451307.
- Knoblauch, R.; Geddes, C.D. Carbon Nanodots in Photodynamic Antimicrobial Therapy: A Review. Materials 2020, 13, 4004.
- Moniruzzaman, M.; Lakshmi, B.A.; Kim, S.; Kim, J. Preparation of Shape-Specific (Trilateral and Quadrilateral) Carbon Quantum Dots towards Multiple Color Emission. Nanoscale 2020, 12, 11947–11959.
- Dong, X.; Ge, L.; Abu Rabe, D.I.; Mohammed, O.O.; Wang, P.; Tang, Y.; Kathariou, S.; Yang, L.; Sun, Y.-P. Photoexcited State Properties and Antibacterial Activities of Carbon Dots Relevant to Mechanistic Features and Implications. Carbon 2020, 170, 137–145.
- Knoblauch, R.; Harvey, A.; Geddes, C.D. Metal-Enhanced Photosensitization of Singlet Oxygen (ME1O2) from Brominated Carbon Nanodots on Silver Nanoparticle Substrates. Plasmonics 2021, 16, 1765–1772.
- Kováčová, M.; Špitalská, E.; Markovic, Z.; Špitálský, Z. Carbon Quantum Dots As Antibacterial Photosensitizers and Their Polymer Nanocomposite Applications. Part. Part. Syst. Charact. 2020, 37, 1900348.
- Marković, Z.M.; Kováčová, M.; Humpolíček, P.; Budimir, M.D.; Vajďák, J.; Kubát, P.; Mičušík, M.; Švajdlenková, H.; Danko, M.; Capáková, Z.; et al. Antibacterial Photodynamic Activity of Carbon Quantum Dots/Polydimethylsiloxane Nanocomposites against Staphylococcus Aureus, Escherichia Coli and Klebsiella Pneumoniae. Photodiagn. Photodyn. Ther. 2019, 26, 342–349.
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-Doped and Hybrid Carbon Dots: A Comprehensive Review on Their Synthesis and Biomedical Applications. J. Control. Release 2021, 330, 132–150.
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent Carbon Dots for Highly Efficient Oxygen Photosensitization and as Photo-Oxidative Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814.
- Dong, Y.; Lin, J.; Chen, Y.; Fu, F.; Chi, Y.; Chen, G. Graphene Quantum Dots, Graphene Oxide, Carbon Quantum Dots and Graphite Nanocrystals in Coals. Nanoscale 2014, 6, 7410–7415.
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene Quantum Dots from Chemistry to Applications. Mater. Today Chem. 2018, 10, 221–258.
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon Dots: Current Advances in Pathogenic Bacteria Monitoring and Prospect Applications. Biosens. Bioelectron. 2020, 156, 112085.
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene Oxide and Carbon Dots as Broad-Spectrum Antimicrobial Agents—A Minireview. Nanoscale Horiz. 2019, 4, 117–137.
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1701406.
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446.
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic Antibacterial Effect of Graphene Quantum Dots. Biomaterials 2014, 35, 4428–4435.
- Sen, P.; Nwahara, N.; Nyokong, T. Photodynamic Antimicrobial Activity of Benzimidazole Substituted Phthalocyanine When Conjugated to Nitrogen Doped Graphene Quantum Dots against Staphylococcus Aureus. Main Group Chem. 2021, 20, 175–191.
- Yu, Y.; Mei, L.; Shi, Y.; Zhang, X.; Cheng, K.; Cao, F.; Zhang, L.; Xu, J.; Li, X.; Xu, Z. Ag-Conjugated Graphene Quantum Dots with Blue Light-Enhanced Singlet Oxygen Generation for Ternary-Mode Highly-Efficient Antimicrobial Therapy. J. Mater. Chem. B 2020, 8, 1371–1382.
- Ferro, S.; Ricchelli, F.; Mancini, G.; Tognon, G.; Jori, G. Inactivation of Methicillin-Resistant Staphylococcus Aureus (MRSA) by Liposome-Delivered Photosensitising Agents. J. Photochem. Photobiol. B Biol. 2006, 83, 98–104.
- Jeong, S.; Lee, J.; Im, B.N.; Park, H.; Na, K. Combined Photodynamic and Antibiotic Therapy for Skin Disorder via Lipase-Sensitive Liposomes with Enhanced Antimicrobial Performance. Biomaterials 2017, 141, 243–250.
- Miretti, M.; Juri, L.; Cosiansi, M.C.; Tempesti, T.C.; Baumgartner, M.T. Antimicrobial Effects of ZnPc Delivered into Liposomes on Multidrug Resistant (MDR)-Mycobacterium Tuberculosis. ChemistrySelect 2019, 4, 9726–9730.
- Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Hypericin Inclusion Complexes Encapsulated in Liposomes for Antimicrobial Photodynamic Therapy. Int. J. Pharm. 2019, 570, 118666.
- Sobotta, L.; Dlugaszewska, J.; Ziental, D.; Szczolko, W.; Koczorowski, T.; Goslinski, T.; Mielcarek, J. Optical Properties of a Series of Pyrrolyl-Substituted Porphyrazines and Their Photoinactivation Potential against Enterococcus Faecalis after Incorporation into Liposomes. J. Photochem. Photobiol. A Chem. 2019, 368, 104–109.
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical Properties and Photocytotoxicity of Free and Liposome-Entrapped Diazepinoporphyrazines on LNCaP Cells under Normoxic and Hypoxic Conditions. Eur. J. Med. Chem. 2018, 150, 64–73.
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341.
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855.
- Park, H.; Lee, J.; Jeong, S.; Im, B.N.; Kim, M.-K.; Yang, S.-G.; Na, K. Lipase-Sensitive Transfersomes Based on Photosensitizer/Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne. Adv. Healthc. Mater. 2016, 5, 3139–3147.
- Wang, Y.; Song, J.; Zhang, F.; Zeng, K.; Zhu, X. Antifungal Photodynamic Activity of Hexyl-Aminolevulinate Ethosomes Against Candida Albicans Biofilm. Front. Microbiol. 2020, 11, 2052.
- Caruso, E.; Orlandi, V.T.; Malacarne, M.C.; Martegani, E.; Scanferla, C.; Pappalardo, D.; Vigliotta, G.; Izzo, L. Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus Aureus. Coatings 2021, 11, 223.
- Castro, K.A.D.F.; Costa, L.D.; Prandini, J.A.; Biazzotto, J.C.; Tomé, A.C.; Hamblin, M.R.; da Graça, P.M.S. Neves, M.; Faustino, M.A.F.; da Silva, R.S. The Photosensitizing Efficacy of Micelles Containing a Porphyrinic Photosensitizer and KI against Resistant Melanoma Cells. Chem.—A Eur. J. 2021, 27, 1990–1994.
- Dantas Lopes dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin-Loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-Mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088.
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586.
- Do Bonfim, C.M.; Monteleoni, L.F.; Calmon, M.d.F.; Cândido, N.M.; Provazzi, P.J.S.; de Souza Lino, V.; Rabachini, T.; Sichero, L.; Villa, L.L.; Quintana, S.M.; et al. Antiviral Activity of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Vulvar Cell Lines Transducing Different Variants of HPV-16. Artif. Cells Nanomed. Biotechnol. 2020, 48, 515–524.
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278.
- Zhu, S.; Wang, X.; Yang, Y.; Bai, H.; Cui, Q.; Sun, H.; Li, L.; Wang, S. Conjugated Polymer with Aggregation-Directed Intramolecular Förster Resonance Energy Transfer Enabling Efficient Discrimination and Killing of Microbial Pathogens. Chem. Mater. 2018, 30, 3244–3253.
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 1700614.
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-Enhanced Two-Photon Photosensitization for Precise Photodynamic Therapy. ACS Nano 2019, 13, 3095–3105.
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951.
- Xu, Q.; He, P.; Wang, J.; Chen, H.; Lv, F.; Liu, L.; Wang, S.; Yoon, J. Antimicrobial Activity of a Conjugated Polymer with Cationic Backbone. Dye. Pigment. 2019, 160, 519–523.
- Zhang, G.; Zhang, D. New Photosensitizer Design Concept: Polymerization-Enhanced Photosensitization. Chem 2018, 4, 2013–2015.
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956.
- Huang, L.; Zhiyentayev, T.; Xuan, Y.; Azhibek, D.; Kharkwal, G.B.; Hamblin, M.R. Photodynamic Inactivation of Bacteria Using Polyethylenimine–Chlorin(E6) Conjugates: Effect of Polymer Molecular Weight, Substitution Ratio of Chlorin(E6) and PH. Lasers Surg. Med. 2011, 43, 313–323.
- Gavara, R.; de Llanos, R.; Pérez-Laguna, V.; Arnau del Valle, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. Broad-Spectrum Photo-Antimicrobial Polymers Based on Cationic Polystyrene and Rose Bengal. Front. Med. 2021, 8, 641646.
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50, 33–44.
- Ahmetali, E.; Sen, P.; Süer, N.C.; Aksu, B.; Nyokong, T.; Eren, T.; Şener, M.K. Enhanced Light-Driven Antimicrobial Activity of Cationic Poly(Oxanorbornene)s by Phthalocyanine Incorporation into Polymer as Pendants. Macromol. Chem. Phys. 2020, 221, 2000386.
- Li, T.; Yan, L. Functional Polymer Nanocarriers for Photodynamic Therapy. Pharmaceuticals 2018, 11, 133.
- Sun, C.-Y.; Shen, S.; Xu, C.-F.; Li, H.-J.; Liu, Y.; Cao, Z.-T.; Yang, X.-Z.; Xia, J.-X.; Wang, J. Tumor Acidity-Sensitive Polymeric Vector for Active Targeted SiRNA Delivery. J. Am. Chem. Soc. 2015, 137, 15217–15224.
- Zhou, J.; Li, T.; Zhang, C.; Xiao, J.; Cui, D.; Cheng, Y. Charge-Switchable Nanocapsules with Multistage PH-Responsive Behaviours for Enhanced Tumour-Targeted Chemo/Photodynamic Therapy Guided by NIR/MR Imaging. Nanoscale 2018, 10, 9707–9719.
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342.
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261.
- Svenson, S. Dendrimers as Versatile Platform in Drug Delivery Applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462.
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774.
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Biological Properties of Water-Soluble Phosphorhydrazone Dendrimers. Braz. J. Pharm. Sci. 2013, 49, 33–44.
- Staegemann, M.H.; Gräfe, S.; Gitter, B.; Achazi, K.; Quaas, E.; Haag, R.; Wiehe, A. Hyperbranched Polyglycerol Loaded with (Zinc-)Porphyrins: Photosensitizer Release Under Reductive and Acidic Conditions for Improved Photodynamic Therapy. Biomacromolecules 2018, 19, 222–238.
- Staegemann, M.H.; Gitter, B.; Dernedde, J.; Kuehne, C.; Haag, R.; Wiehe, A. Mannose-Functionalized Hyperbranched Polyglycerol Loaded with Zinc Porphyrin: Investigation of the Multivalency Effect in Antibacterial Photodynamic Therapy. Chem.—A Eur. J. 2017, 23, 3918–3930.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Kubát, P.; Henke, P.; Raya, R.K.; Štěpánek, M.; Mosinger, J. Polystyrene and Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Nanoparticles with Porphyrins: Structure, Size, and Photooxidation Properties. Langmuir 2020, 36, 302–310.
- Gualdesi, M.S.; Vara, J.; Aiassa, V.; Alvarez Igarzabal, C.I.; Ortiz, C.S. New Poly(Acrylamide) Nanoparticles in the Development of Third Generation Photosensitizers. Dye. Pigment. 2021, 184, 108856.
- Malá, Z.; Žárská, L.; Malina, L.; Langová, K.; Večeřová, R.; Kolář, M.; Henke, P.; Mosinger, J.; Kolářová, H. Photodynamic Effect of TPP Encapsulated in Polystyrene Nanoparticles toward Multi-Resistant Pathogenic Bacterial Strains: AFM Evaluation. Sci. Rep. 2021, 11, 6786.
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.-A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear Bond Strength, Adhesive Remnant Index, and Anti-Biofilm Effects of a Photoexcited Modified Orthodontic Adhesive Containing Curcumin Doped Poly Lactic-Co-Glycolic Acid Nanoparticles: An Ex-Vivo Biofilm Model of S. Mutans on the Enamel Slab Bonded Brackets. Photodiagn. Photodyn. Ther. 2020, 30, 101674.
- Liang, Y.; Zhang, H.; Yuan, H.; Lu, W.; Li, Z.; Wang, L.; Gao, L.-H. Conjugated Polymer and Triphenylamine Derivative Codoped Nanoparticles for Photothermal and Photodynamic Antimicrobial Therapy. ACS Appl. Bio. Mater. 2020, 3, 3494–3499.
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, e1900301.
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121.
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Banerjee, U.C. Porphyrin Functionalized Gelatin Nanoparticle-Based Biodegradable Phototheranostics: Potential Tools for Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 4202–4212.
- Barbosa, P.M.; Campanholi, K.d.S.S.; Vilsinski, B.H.; Ferreira, S.B.d.S.; Gonçalves, R.S.; de Castro-Hoshino, L.V.; de Oliveira, A.C.V.; Sato, F.; Baesso, M.L.; Bruschi, M.L.; et al. Aluminum Phthalocyanine Hydroxide-Loaded Thermoresponsive Biomedical Hydrogel: A Design for Targeted Photosensitizing Drug Delivery. J. Mol. Liq. 2021, 341, 117421.
- Zheng, Y.; Yu, E.; Weng, Q.; Zhou, L.; Li, Q. Optimization of Hydrogel Containing Toluidine Blue O for Photodynamic Therapy in Treating Acne. Lasers Med. Sci. 2019, 34, 1535–1545.
- Feng, Y.; Xiao, K.; He, Y.; Du, B.; Hong, J.; Yin, H.; Lu, D.; Luo, F.; Li, Z.; Li, J.; et al. Tough and Biodegradable Polyurethane-Curcumin Composited Hydrogel with Antioxidant, Antibacterial and Antitumor Properties. Mater. Sci. Eng. C 2021, 121, 111820.
- de Souza, C.M.; Garcia, M.T.; de Barros, P.P.; Pedroso, L.L.C.; Ward, R.A.d.C.; Strixino, J.F.; Melo, V.M.M.; Junqueira, J.C. Chitosan Enhances the Antimicrobial Photodynamic Inactivation Mediated by Photoditazine® against Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2020, 32, 102001.
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502637.
- Pourhajibagher, M.; Rokn, A.R.; Rostami-Rad, M.; Barikani, H.R.; Bahador, A. Monitoring of Virulence Factors and Metabolic Activity in Aggregatibacter Actinomycetemcomitans Cells Surviving Antimicrobial Photodynamic Therapy via Nano-Chitosan Encapsulated Indocyanine Green. Front. Phys. 2018, 6, 124.
- Rad, M.R.; Pourhajibagher, M.; Rokn, A.R.; Barikani, H.R.; Bahador, A. Effect of Antimicrobial Photodynamic Therapy Using Indocyanine Green Doped with Chitosan Nanoparticles on Biofilm Formation-Related Gene Expression of Aggregatibacter Actinomycetemcomitans. Front. Dent. 2019, 16, 187–193.
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy. Photochem. Photobiol. 2012, 88, 577–583.
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26711–26721.
- Rizzi, V.; Fini, P.; Fanelli, F.; Placido, T.; Semeraro, P.; Sibillano, T.; Fraix, A.; Sortino, S.; Agostiano, A.; Giannini, C.; et al. Molecular Interactions, Characterization and Photoactivity of Chlorophyll a/Chitosan/2-HP-β-Cyclodextrin Composite Films as Functional and Active Surfaces for ROS Production. Food Hydrocoll. 2016, 58, 98–112.
- Sharma, M.; Dube, A.; Majumder, S.K. Antibacterial Photodynamic Activity of Photosensitizer-Embedded Alginate-Pectin-Carboxymethyl Cellulose Composite Biopolymer Films. Lasers Med. Sci. 2021, 36, 763–772.
- Hasanin, M.S.; Abdelraof, M.; Fikry, M.; Shaker, Y.M.; Sweed, A.M.K.; Senge, M.O. Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)Porphyrin. Molecules 2021, 26, 3551.
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/BFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169.
- Müller, A.; Preuß, A.; Bornhütter, T.; Thomas, I.; Prager, A.; Schulze, A.; Röder, B. Electron Beam Functionalized Photodynamic Polyethersulfone Membranes—Photophysical Characterization and Antimicrobial Activity. Photochem. Photobiol. Sci. 2018, 17, 1346–1354.
- Martínez, S.R.; Palacios, Y.B.; Heredia, D.A.; Aiassa, V.; Bartolilla, A.; Durantini, A.M. Self-Sterilizing 3D-Printed Polylactic Acid Surfaces Coated with a BODIPY Photosensitizer. ACS Appl. Mater. Interfaces 2021, 13, 11597–11608.
- Santos, M.R.E.; Mendonça, P.V.; Branco, R.; Sousa, R.; Dias, C.; Serra, A.C.; Fernandes, J.R.; Magalhães, F.D.; Morais, P.V.; Coelho, J.F.J. Light-Activated Antimicrobial Surfaces Using Industrial Varnish Formulations to Mitigate the Incidence of Nosocomial Infections. ACS Appl. Mater. Interfaces 2021, 13, 7567–7579.
- Sautrot-Ba, P.; Contreras, A.; Andaloussi, S.A.; Coradin, T.; Hélary, C.; Razza, N.; Sangermano, M.; Mazeran, P.-E.; Malval, J.-P.; Versace, D.-L. Eosin-Mediated Synthesis of Polymer Coatings Combining Photodynamic Inactivation and Antimicrobial Properties. J. Mater. Chem. B 2017, 5, 7572–7582.
- Kováčová, M.; Kleinová, A.; Vajďák, J.; Humpolíček, P.; Kubát, P.; Bodík, M.; Marković, Z.; Špitálský, Z. Photodynamic-Active Smart Biocompatible Material for an Antibacterial Surface Coating. J. Photochem. Photobiol. B Biol. 2020, 211, 112012.
- Wylie, M.P.; Irwin, N.J.; Howard, D.; Heydon, K.; McCoy, C.P. Hot-Melt Extrusion of Photodynamic Antimicrobial Polymers for Prevention of Microbial Contamination. J. Photochem. Photobiol. B Biol. 2021, 214, 112098.
- Moor, K.J.; Osuji, C.O.; Kim, J.-H. Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces 2016, 8, 33583–33591.
- Mafukidze, D.M.; Sindelo, A.; Nyokong, T. Spectroscopic Characterization and Photodynamic Antimicrobial Chemotherapy of Phthalocyanine-Silver Triangular Nanoprism Conjugates When Supported on Asymmetric Polymer Membranes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 333–345.
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L. Self-Enriched Mesoporous Silica Nanoparticle Composite Membrane with Remarkable Photodynamic Antimicrobial Performances. J. Colloid Interface Sci. 2020, 559, 197–205.
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587.
- Peddinti, B.S.T.; Scholle, F.; Ghiladi, R.A.; Spontak, R.J. Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. ACS Appl. Mater. Interfaces 2018, 10, 25955–25959.
- Baigorria, E.; Milanesio, M.E.; Durantini, E.N. Synthesis, Spectroscopic Properties and Photodynamic Activity of Zn(II) Phthalocyanine-Polymer Conjugates as Antimicrobial Agents. Eur. Polym. J. 2020, 134, 109816.
- Ambrósio, J.A.R.; Pinto, B.C.D.S.; da Silva, B.G.M.; Passos, J.C.d.S.; Beltrame Junior, M.; Costa, M.S.; Simioni, A.R. BSA Nanoparticles Loaded-Methylene Blue for Photodynamic Antimicrobial Chemotherapy (PACT): Effect on Both Growth and Biofilm Formation by Candida Albicans. J. Biomater. Sci. Polym. Ed. 2020, 31, 2182–2198.
- Silva, E.P.O.; Ribeiro, N.M.; Cardoso, M.A.G.; Pacheco-Soares, C.; Jr, M.B. Photodynamic Therapy Using Silicon Phthalocyanine Conjugated with Bovine Serum Albumin as a Drug Delivery System. Laser Phys. 2021, 31, 075601.
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657.
- Dolanský, J.; Henke, P.; Malá, Z.; Žárská, L.; Kubát, P.; Mosinger, J. Antibacterial Nitric Oxide- and Singlet Oxygen-Releasing Polystyrene Nanoparticles Responsive to Light and Temperature Triggers. Nanoscale 2018, 10, 2639–2648.
- Ren, B.; Li, K.; Liu, Z.; Liu, G.; Wang, H. White Light-Triggered Zwitterionic Polymer Nanoparticles Based on an AIE-Active Photosensitizer for Photodynamic Antimicrobial Therapy. J. Mater. Chem. B 2020, 8, 10754–10763.
- Wang, H.; He, J.; Zhang, M.; Tao, Y.; Li, F.; Tam, K.C.; Ni, P. Biocompatible and Acid-Cleavable Poly(ε-Caprolactone)-Acetal-Poly(Ethylene Glycol)-Acetal-Poly(ε-Caprolactone) Triblock Copolymers: Synthesis, Characterization and PH-Triggered Doxorubicin Delivery. J. Mater. Chem. B 2013, 1, 6596–6607.
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-Responsive Polymeric Micelles for Drug Delivery and Cancer Therapy. IJN 2018, 13, 2921–2942.
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985.
- Shen, H.; Eisenberg, A. Morphological Phase Diagram for a Ternary System of Block Copolymer PS310-b-PAA52/Dioxane/H2O. J. Phys. Chem. B 1999, 103, 9473–9487.
- Zhang, L.; Eisenberg, A. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-Poly(Acrylic Acid) Block Copolymers. Science 1995, 268, 1728–1731.
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, e3702518.
- Won, Y.-Y.; Brannan, A.K.; Davis, H.T.; Bates, F.S. Cryogenic Transmission Electron Microscopy (Cryo-TEM) of Micelles and Vesicles Formed in Water by Poly(Ethylene Oxide)-Based Block Copolymers. J. Phys. Chem. B 2002, 106, 3354–3364.
- Haas, S.; Chen, Y.; Fuchs, C.; Handschuh, S.; Steuber, M.; Schönherr, H. Amphiphilic Block Copolymer Vesicles for Active Wound Dressings: Synthesis of Model Systems and Studies of Encapsulation and Release. Macromol. Symp. 2013, 328, 73–79.
- Haas, S.; Hain, N.; Raoufi, M.; Handschuh-Wang, S.; Wang, T.; Jiang, X.; Schönherr, H. Enzyme Degradable Polymersomes from Hyaluronic Acid-Block-Poly(ε-Caprolactone) Copolymers for the Detection of Enzymes of Pathogenic Bacteria. Biomacromolecules 2015, 16, 832–841.
- Handschuh-Wang, S.; Wesner, D.; Wang, T.; Lu, P.; Tücking, K.-S.; Haas, S.; Druzhinin, S.I.; Jiang, X.; Schönherr, H. Determination of the Wall Thickness of Block Copolymer Vesicles by Fluorescence Lifetime Imaging Microscopy. Macromol. Chem. Phys. 2017, 218, 1600454.
- Tücking, K.-S.; Handschuh-Wang, S.; Schönherr, H.; Tücking, K.-S.; Handschuh-Wang, S.; Schönherr, H. Bacterial Enzyme Responsive Polymersomes: A Closer Look at the Degradation Mechanism of PEG-Block-PLA Vesicles. Aust. J. Chem. 2014, 67, 578–584.
- Tang, Q.; Hu, P.; Peng, H.; Zhang, N.; Zheng, Q.; He, Y. Near-Infrared Laser-Triggered, Self-Immolative Smart Polymersomes for in Vivo Cancer Therapy. Int. J. Nanomed. 2020, 15, 137–149.
- Knop, K.; Mingotaud, A.-F.; El-Akra, N.; Violleau, F.; Souchard, J.-P. Monomeric Pheophorbide(a)-Containing Poly(Ethyleneglycol-b-ε-Caprolactone) Micelles for Photodynamic Therapy. Photochem. Photobiol. Sci. 2009, 8, 396–404.
- Zhang, G.-D.; Harada, A.; Nishiyama, N.; Jiang, D.-L.; Koyama, H.; Aida, T.; Kataoka, K. Polyion Complex Micelles Entrapping Cationic Dendrimer Porphyrin: Effective Photosensitizer for Photodynamic Therapy of Cancer. J. Control. Release 2003, 93, 141–150.
- Xiao-ying, Z.; Pei-ying, Z. Polymersomes in Nanomedicine—A Review. Curr. Nanosci. 2017, 13, 124–129.
- Lanzilotto, A.; Kyropoulou, M.; Constable, E.C.; Housecroft, C.E.; Meier, W.P.; Palivan, C.G. Porphyrin-Polymer Nanocompartments: Singlet Oxygen Generation and Antimicrobial Activity. J. Biol. Inorg. Chem. 2018, 23, 109–122.
- Li, Z.; Fan, F.; Ma, J.; Yin, W.; Zhu, D.; Zhang, L.; Wang, Z. Oxygen- and Bubble-Generating Polymersomes for Tumor-Targeted and Enhanced Photothermal–Photodynamic Combination Therapy. Biomater. Sci. 2021, 9, 5841–5853.
- Avci, P.; Erdem, S.S.; Hamblin, M.R. Photodynamic Therapy: One Step Ahead with Self-Assembled Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1937–1952.
- Gibot, L.; Demazeau, M.; Pimienta, V.; Mingotaud, A.-F.; Vicendo, P.; Collin, F.; Martins-Froment, N.; Dejean, S.; Nottelet, B.; Roux, C.; et al. Role of Polymer Micelles in the Delivery of Photodynamic Therapy Agent to Liposomes and Cells. Cancers 2020, 12, 384.
- Karges, J.; Tharaud, M.; Gasser, G. Polymeric Encapsulation of a Ru(II)-Based Photosensitizer for Folate-Targeted Photodynamic Therapy of Drug Resistant Cancers. J. Med. Chem. 2021, 64, 4612–4622.
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in Antimicrobial Photodynamic Inactivation at the Nanoscale. Nanophotonics 2017, 6, 853–879.
- Vilsinski, B.H.; Gerola, A.P.; Enumo, J.A.; Campanholi, K.d.S.S.; Pereira, P.C.d.S.; Braga, G.; Hioka, N.; Kimura, E.; Tessaro, A.L.; Caetano, W. Formulation of Aluminum Chloride Phthalocyanine in Pluronic(TM) P-123 and F-127 Block Copolymer Micelles: Photophysical Properties and Photodynamic Inactivation of Microorganisms. Photochem. Photobiol. 2015, 91, 518–525.
- Tsai, T.; Yang, Y.-T.; Wang, T.-H.; Chien, H.-F.; Chen, C.-T. Improved Photodynamic Inactivation of Gram-Positive Bacteria Using Hematoporphyrin Encapsulated in Liposomes and Micelles. Lasers Surg. Med. 2009, 41, 316–322.
- Teng, F.; Deng, P.; Song, Z.; Zhou, F.; Feng, R. Enhanced Effect in Combination of Curcumin- and Ketoconazole-Loaded Methoxy Poly (Ethylene Glycol)-Poly (ε-Caprolactone) Micelles. Biomed. Pharmacother. 2017, 88, 43–51.
- Yi, X.; Hu, J.-J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-Guiding Polymeric Prodrug Micelles with Two Aggregation-Induced Emission Photosensitizers for Enhanced Chemo-Photodynamic Therapy. ACS Nano 2021, 15, 3026–3037.
- Demir, B.; Barlas, F.B.; Gumus, Z.P.; Unak, P.; Timur, S. Theranostic Niosomes as a Promising Tool for Combined Therapy and Diagnosis: “All-in-One” Approach. ACS Appl. Nano Mater. 2018, 1, 2827–2835.
- Fadel, M.A.; Tawfik, A.A. New Topical Photodynamic Therapy for Treatment of Hidradenitis Suppurativa Using Methylene Blue Niosomal Gel: A Single-Blind, Randomized, Comparative Study. Clin. Exp. Dermatol. 2015, 40, 116–122.
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Novel Nanostructured Smart, Photodynamic Hydrogels Based on Poly(N-Isopropylacrylamide) Bearing Porphyrin Units in Their Crosslink Chains: A Potential Sensitizer System in Cancer Therapy. Polymer 2017, 109, 93–105.
- Preis, E.; Anders, T.; Širc, J.; Hobzova, R.; Cocarta, A.-I.; Bakowsky, U.; Jedelská, J. Biocompatible Indocyanine Green Loaded PLA Nanofibers for In Situ Antimicrobial Photodynamic Therapy. Mater. Sci. Eng. C 2020, 115, 111068.
- Stoll, K.R.; Scholle, F.; Zhu, J.; Zhang, X.; Ghiladi, R.A. BODIPY-Embedded Electrospun Materials in Antimicrobial Photodynamic Inactivation. Photochem. Photobiol. Sci. 2019, 18, 1923–1932.
- Chen, W.; Chen, J.; Li, L.; Wang, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Wool/Acrylic Blended Fabrics as Next-Generation Photodynamic Antimicrobial Materials. ACS Appl. Mater. Interfaces 2019, 11, 29557–29568.
- Contreras, A.; Raxworthy, M.J.; Wood, S.; Schiffman, J.D.; Tronci, G. Photodynamically Active Electrospun Fibers for Antibiotic-Free Infection Control. ACS Appl. Bio Mater. 2019, 2, 4258–4270.
- Czapka, T.; Winkler, A.; Maliszewska, I.; Kacprzyk, R. Fabrication of Photoactive Electrospun Cellulose Acetate Nanofibers for Antibacterial Applications. Energies 2021, 14, 2598.
- Managa, M.; Amuhaya, E.K.; Nyokong, T. Photodynamic Antimicrobial Chemotherapy Activity of (5,10,15,20-Tetrakis(4-(4-Carboxyphenycarbonoimidoyl)Phenyl)Porphyrinato) Chloro Gallium(III). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 867–874.
- Sindelo, A.; Nyokong, T. Magnetic Nanoparticle—Indium Phthalocyanine Conjugate Embedded in Electrospun Fiber for Photodynamic Antimicrobial Chemotherapy and Photodegradation of Methyl Red. Heliyon 2019, 5, e02352.
- Miñán, A.; Lorente, C.; Ipiña, A.; Thomas, A.H.; Fernández Lorenzo de Mele, M.; Schilardi, P.L. Photodynamic Inactivation Induced by Carboxypterin: A Novel Non-Toxic Bactericidal Strategy against Planktonic Cells and Biofilms of Staphylococcus Aureus. Biofouling 2015, 31, 459–468.
- Tosato, M.G.; Schilardi, P.; Lorenzo de Mele, M.F.; Thomas, A.H.; Lorente, C.; Miñán, A. Synergistic Effect of Carboxypterin and Methylene Blue Applied to Antimicrobial Photodynamic Therapy against Mature Biofilm of Klebsiella Pneumoniae. Heliyon 2020, 6, e03522.
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual Wavelength Irradiation Antimicrobial Photodynamic Therapy Using Indocyanine Green and Metformin Doped with Nano-Curcumin as an Efficient Adjunctive Endodontic Treatment Modality. Photodiagn. Photodyn. Ther. 2020, 29, 101628.
- Openda, Y.I.; Sen, P.; Managa, M.; Nyokong, T. Acetophenone Substituted Phthalocyanines and Their Graphene Quantum Dots Conjugates as Photosensitizers for Photodynamic Antimicrobial Chemotherapy against Staphylococcus Aureus. Photodiagn. Photodyn. Ther. 2020, 29, 101607.
- Hamblin, M.R. Potentiation of Antimicrobial Photodynamic Inactivation by Inorganic Salts. Expert Rev. Anti-Infect. Ther. 2017, 15, 1059–1069.
- Huang, L.; St. Denis, T.G.; Xuan, Y.; Huang, Y.-Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical Potentiation of Methylene Blue-Mediated Antimicrobial Photodynamic Inactivation by Sodium Azide: Role of Ambient Oxygen and Azide Radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071.
- Kasimova, K.R.; Sadasivam, M.; Landi, G.; Sarna, T.; Hamblin, M.R. Potentiation of Photoinactivation of Gram-Positive and Gram-Negative Bacteria Mediated by Six Phenothiazinium Dyes by Addition of Azide Ion. Photochem. Photobiol. Sci. 2014, 13, 1541–1548.
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212.
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on APDT Efficacy. Front. Microbiol. 2018, 9, 2665.
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic Inactivation of Microorganisms Sensitized by Cationic BODIPY Derivatives Potentiated by Potassium Iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536.
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin–Schiff Base Conjugates Bearing Basic Amino Groups as Antimicrobial Phototherapeutic Agents. Molecules 2021, 26, 5877.
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190.
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of Divalent Cations, EDTA and Chitosan on the Uptake and Photoinactivation of Escherichia Coli Mediated by Cationic and Anionic Porphyrins. Photodiagn. Photodyn. Ther. 2015, 12, 67–75.
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A Combination of Photodynamic Therapy and Antimicrobial Compounds to Treat Skin and Mucosal Infections: A Systematic Review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029.
- Barra, F.; Roscetto, E.; Soriano, A.A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.M.; Palumbo, G.; Catania, M.R. Photodynamic and Antibiotic Therapy in Combination to Fight Biofilms and Resistant Surface Bacterial Infections. Int. J. Mol. Sci. 2015, 16, 20417–20430.
- Cahan, R.; Swissa, N.; Gellerman, G.; Nitzan, Y. Photosensitizer–Antibiotic Conjugates: A Novel Class of Antibacterial Molecules. Photochem. Photobiol. 2010, 86, 418–425.
- Lu, Z.; Dai, T.; Huang, L.; Kurup, D.B.; Tegos, G.P.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Photodynamic Therapy with a Cationic Functionalized Fullerene Rescues Mice from Fatal Wound Infections. Nanomedicine 2010, 5, 1525–1533.
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas Aeruginosa Biofilms. Curr. Microbiol. 2010, 61, 411–416.
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic Inactivation of Multidrug-Resistant Bacteria in Hospital Wastewaters: Influence of Residual Antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626.
- Tavares, L.J.; de Avila, E.D.; Klein, M.I.; Panariello, B.H.D.; Spolidório, D.M.P.; Pavarina, A.C. Antimicrobial Photodynamic Therapy Alone or in Combination with Antibiotic Local Administration against Biofilms of Fusobacterium Nucleatum and Porphyromonas Gingivalis. J. Photochem. Photobiol. B Biol. 2018, 188, 135–145.
- Di Poto, A.; Sbarra, M.S.; Provenza, G.; Visai, L.; Speziale, P. The Effect of Photodynamic Treatment Combined with Antibiotic Action or Host Defence Mechanisms on Staphylococcus Aureus Biofilms. Biomaterials 2009, 30, 3158–3166.
- Feng, Y.; Palanisami, A.; Ashraf, S.; Bhayana, B.; Hasan, T. Photodynamic Inactivation of Bacterial Carbapenemases Restores Bacterial Carbapenem Susceptibility and Enhances Carbapenem Antibiotic Effectiveness. Photodiagn. Photodyn. Ther. 2020, 30, 101693.
- Tanaka, M.; Mroz, P.; Dai, T.; Huang, L.; Morimoto, Y.; Kinoshita, M.; Yoshihara, Y.; Shinomiya, N.; Seki, S.; Nemoto, K.; et al. Linezolid and Vancomycin Decrease the Therapeutic Effect of Methylene Blue-Photodynamic Therapy in a Mouse Model of MRSA Bacterial Arthritis. Photochem. Photobiol. 2013, 89, 679–682.
- Jiang, Q.; Fangjie, E.; Tian, J.; Yang, J.; Zhang, J.; Cheng, Y. Light-Excited Antibiotics for Potentiating Bacterial Killing via Reactive Oxygen Species Generation. ACS Appl. Mater. Interfaces 2020, 12, 16150–16158.
- Morschhäuser, J. The Development of Fluconazole Resistance in Candida Albicans—An Example of Microevolution of a Fungal Pathogen. J. Microbiol. 2016, 54, 192–201.
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic Inactivation of Candida Albicans by a Tetracationic Tentacle Porphyrin and Its Analogue without Intrinsic Charges in Presence of Fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340.
- Hu, Y.; Huang, X.; Lu, S.; Hamblin, M.R.; Mylonakis, E.; Zhang, J.; Xi, L. Photodynamic Therapy Combined with Terbinafine Against Chromoblastomycosis and the Effect of PDT on Fonsecaea Monophora In Vitro. Mycopathologia 2015, 179, 103–109.
- Hu, Y.; Qi, X.; Sun, H.; Lu, Y.; Hu, Y.; Chen, X.; Liu, K.; Yang, Y.; Mao, Z.; Wu, Z.; et al. Photodynamic Therapy Combined with Antifungal Drugs against Chromoblastomycosis and the Effect of ALA-PDT on Fonsecaea In Vitro. PLoS Negl. Trop. Dis. 2019, 13, e0007849.
- de Freitas, L.M.; Lorenzón, E.N.; Cilli, E.M.; de Oliveira, K.T.; Fontana, C.R.; Mang, T.S. Photodynamic and Peptide-Based Strategy to Inhibit Gram-Positive Bacterial Biofilm Formation. Biofouling 2019, 35, 742–757.
- Chen, H.; Li, S.; Wu, M.; Kenry; Huang, Z.; Lee, C.; Liu, B. Membrane-Anchoring Photosensitizer with Aggregation-Induced Emission Characteristics for Combating Multidrug-Resistant Bacteria. Angew. Chem. Int. Ed. 2020, 59, 632–636.
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal Bioconjugate for Targeted Gram-Negative Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852.
- Leanse, L.G.; Dong, P.-T.; Goh, X.S.; Lu, M.; Cheng, J.-X.; Hooper, D.C.; Dai, T. Quinine Enhances Photo-Inactivation of Gram-Negative Bacteria. J. Infect. Dis. 2020, 221, 618–626.
- Le Gall, T.; Berchel, M.; Le Hir, S.; Fraix, A.; Salaün, J.Y.; Férec, C.; Lehn, P.; Jaffrès, P.-A.; Montier, T. Arsonium-Containing Lipophosphoramides, Poly-Functional Nano-Carriers for Simultaneous Antibacterial Action and Eukaryotic Cell Transfection. Adv. Healthc. Mater. 2013, 2, 1513–1524.
- Lin, S.; Liu, C.; Han, X.; Zhong, H.; Cheng, C. Viral Nanoparticle System: An Effective Platform for Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 1728.
- Hosseini, N.; Pourhajibagher, M.; Chiniforush, N.; Hosseinkhan, N.; Rezaie, P.; Bahador, A. Modulation of Toxin-Antitoxin System Rnl AB Type II in Phage-Resistant Gammaproteobacteria Surviving Photodynamic Treatment. J. Lasers Med. Sci. 2018, 10, 21–28.
- Dong, S.; Shi, H.; Zhang, X.; Chen, X.; Cao, D.; Mao, C.; Gao, X.; Wang, L. Difunctional Bacteriophage Conjugated with Photosensitizers for Candida Albicans-Targeting Photodynamic Inactivation. Int. J. Nanomed. 2018, 13, 2199–2216.
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680.
- Huang, X.; Chen, G.; Pan, J.; Chen, X.; Huang, N.; Wang, X.; Liu, J. Effective PDT/PTT Dual-Modal Phototherapeutic Killing of Pathogenic Bacteria by Using Ruthenium Nanoparticles. J. Mater. Chem. B 2016, 4, 6258–6270.
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.; de Carvalho Castro e Silva, C.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2020, 10, 2995.
- Liu, Z.; Li, J.; Chen, W.; Liu, L.; Yu, F. Light and Sound to Trigger the Pandora’s Box against Breast Cancer: A Combination Strategy of Sonodynamic, Photodynamic and Photothermal Therapies. Biomaterials 2020, 232, 119685.
- Mai, B.; Wang, X.; Liu, Q.; Zhang, K.; Wang, P. The Application of DVDMS as a Sensitizing Agent for Sono-/Photo-Therapy. Front. Pharm. 2020, 11, 19.
- Fraix, A.; Sortino, S. Combination of PDT Photosensitizers with NO Photodononors. Photochem. Photobiol. Sci. 2018, 17, 1709–1727.
- Dolanský, J.; Henke, P.; Kubát, P.; Fraix, A.; Sortino, S.; Mosinger, J. Polystyrene Nanofiber Materials for Visible-Light-Driven Dual Antibacterial Action via Simultaneous Photogeneration of NO and O2((1)Δg). ACS Appl. Mater. Interfaces 2015, 7, 22980–22989.
- Parisi, C.; Failla, M.; Fraix, A.; Rescifina, A.; Rolando, B.; Lazzarato, L.; Cardile, V.; Graziano, A.C.E.; Fruttero, R.; Gasco, A.; et al. A Molecular Hybrid Producing Simultaneously Singlet Oxygen and Nitric Oxide by Single Photon Excitation with Green Light. Bioorganic Chem. 2019, 85, 18–22.
- Maya, R.; Ladeira, L.L.C.; Maya, J.E.P.; Mail, L.M.G.; Bussadori, S.K.; Paschoal, M.A.B. The Combination of Antimicrobial Photodynamic Therapy and Photobiomodulation Therapy for the Treatment of Palatal Ulcers: A Case Report. J. Lasers Med. Sci. 2020, 11, 228–233.
- Alqerban, A. Efficacy of Antimicrobial Photodynamic and Photobiomodulation Therapy against Treponema Denticola, Fusobacterium Nucleatum and Human Beta Defensin-2 Levels in Patients with Gingivitis Undergoing Fixed Orthodontic Treatment: A Clinic-Laboratory Study. Photodiagn. Photodyn. Ther. 2020, 29, 101659.
- Fekrazad, R. Photobiomodulation and Antiviral Photodynamic Therapy as a Possible Novel Approach in COVID-19 Management. Photobiomodulation Photomed. Laser Surg. 2020, 38, 255–257.
- Lago, A.D.N.; Fortes, A.B.C.; Furtado, G.S.; Menezes, C.F.S.; Gonçalves, L.M. Association of Antimicrobial Photodynamic Therapy and Photobiomodulation for Herpes Simplex Labialis Resolution: Case Series. Photodiagn. Photodyn. Ther. 2020, 32, 102070.
- Teixeira, I.S.; Leal, F.S.; Tateno, R.Y.; Palma, L.F.; Campos, L. Photobiomodulation Therapy and Antimicrobial Photodynamic Therapy for Orofacial Lesions in Patients with COVID-19: A Case Series. Photodiagn. Photodyn. Ther. 2021, 34, 102281.
- Ma, X.; Pan, H.; Wu, G.; Yang, Z.; Yi, J. Ultrasound May Be Exploited for the Treatment of Microbial Diseases. Med. Hypotheses 2009, 73, 18–19.
- Serpe, L.; Giuntini, F. Sonodynamic Antimicrobial Chemotherapy: First Steps towards a Sound Approach for Microbe Inactivation. J. Photochem. Photobiol. B Biol. 2015, 150, 44–49.
- Alves, F.; Pavarina, A.C.; Mima, E.G.d.O.; McHale, A.P.; Callan, J.F. Antimicrobial Sonodynamic and Photodynamic Therapies against Candida Albicans. Biofouling 2018, 34, 357–367.
- Costley, D.; Ewan, C.M.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating Cancer with Sonodynamic Therapy: A Review. Int. J. Hyperth. 2015, 31, 107–117.
- Alves, F.; Gomes Guimarães, G.; Mayumi Inada, N.; Pratavieira, S.; Salvador Bagnato, V.; Kurachi, C. Strategies to Improve the Antimicrobial Efficacy of Photodynamic, Sonodynamic, and Sonophotodynamic Therapies. Lasers Surg. Med. 2021, lsm.23383.
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial Biofilm Modulation by Ultrasound: Current Concepts and Controversies. Ultrason. Sonochemistry 2014, 21, 15–22.
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Using Sound for Microbial Eradication—Light at the End of the Tunnel? FEMS Microbiol. Lett. 2014, 356, 20–22.
- Pourhajibagher, M.; reza Rokn, A.; reza Barikani, H.; Bahador, A. Photo-Sonodynamic Antimicrobial Chemotherapy via Chitosan Nanoparticles-Indocyanine Green against Polymicrobial Periopathogenic Biofilms: Ex Vivo Study on Dental Implants. Photodiagn. Photodyn. Ther. 2020, 31, 101834.
- Pourhajibagher, M.; Bahador, A. Attenuation of Aggregatibacter Actinomycetemcomitans Virulence Using Curcumin-Decorated Nanophytosomes-Mediated Photo-Sonoantimicrobial Chemotherapy. Sci. Rep. 2021, 11, 6012.
- Traitcheva, N.; Berg, H. Electroporation and Alternating Current Cause Membrane Permeation of Photodynamic Cytotoxins Yielding Necrosis and Apoptosis of Cancer Cells. Bioelectrochemistry 2010, 79, 257–260.
- Weżgowiec, J.; Kulbacka, J.; Saczko, J.; Rossowska, J.; Chodaczek, G.; Kotulska, M. Biological Effects in Photodynamic Treatment Combined with Electropermeabilization in Wild and Drug Resistant Breast Cancer Cells. Bioelectrochemistry 2018, 123, 9–18.
- de Melo, W.d.C.M.A.; Lee, A.N.; Perussi, J.R.; Hamblin, M.R. Electroporation Enhances Antimicrobial Photodynamic Therapy Mediated by the Hydrophobic Photosensitizer, Hypericin. Photodiagn. Photodyn. Ther. 2013, 10, 647–650.
- Ayaz, F.; Gonul, İ.; Demirbag, B.; Ocakoglu, K. Differential Immunomodulatory Activities of Schiff Base Complexes Depending on Their Metal Conjugation. Inflammation 2019, 42, 1878–1885.
- Sadraeian, M.; Bahou, C.; da Cruz, E.F.; Janini, L.M.R.; Sobhie Diaz, R.; Boyle, R.W.; Chudasama, V.; Eduardo Gontijo Guimarães, F. Photoimmunotherapy Using Cationic and Anionic Photosensitizer-Antibody Conjugates against HIV Env-Expressing Cells. Int. J. Mol. Sci. 2020, 21, 9151.
- Reinhard, A.; Sandborn, W.J.; Melhem, H.; Bolotine, L.; Chamaillard, M.; Peyrin-Biroulet, L. Photodynamic Therapy as a New Treatment Modality for Inflammatory and Infectious Conditions. Expert Rev. Clin. Immunol. 2015, 11, 637–657.
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 2019, 56, 23–27.
- Prasad, P.; Gupta, A.; Sasmal, P.K. Aggregation-Induced Emission Active Metal Complexes: A Promising Strategy to Tackle Bacterial Infections. Chem. Commun. 2021, 57, 174–186.
- Gao, S.; Yan, X.; Xie, G.; Zhu, M.; Ju, X.; Stang, P.J.; Tian, Y.; Niu, Z. Membrane Intercalation-Enhanced Photodynamic Inactivation of Bacteria by a Metallacycle and TAT-Decorated Virus Coat Protein. Proc. Natl. Acad. Sci. USA 2019, 116, 23437–23443.
- Jerjes, W.; Theodossiou, T.A.; Hirschberg, H.; Høgset, A.; Weyergang, A.; Selbo, P.K.; Hamdoon, Z.; Hopper, C.; Berg, K. Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J. Clin. Med. 2020, 9, 528.
- Zhang, X.; de Boer, L.; Heiliegers, L.; Man-Bovenkerk, S.; Selbo, P.K.; Drijfhout, J.W.; Høgset, A.; Zaat, S.A.J. Photochemical Internalization Enhances Cytosolic Release of Antibiotic and Increases Its Efficacy against Staphylococcal Infection. J. Control. Release 2018, 283, 214–222.
- Hilgers, F.; Bitzenhofer, N.L.; Ackermann, Y.; Burmeister, A.; Grünberger, A.; Jaeger, K.-E.; Drepper, T. Genetically Encoded Photosensitizers as Light-Triggered Antimicrobial Agents. Int. J. Mol. Sci. 2019, 20, 4608.
- Gorbachev, D.A.; Staroverov, D.B.; Lukyanov, K.A.; Sarkisyan, K.S. Genetically Encoded Red Photosensitizers with Enhanced Phototoxicity. Int. J. Mol. Sci. 2020, 21, 8800.
- Endres, S.; Wingen, M.; Torra, J.; Ruiz-González, R.; Polen, T.; Bosio, G.; Bitzenhofer, N.L.; Hilgers, F.; Gensch, T.; Nonell, S.; et al. An Optogenetic Toolbox of LOV-Based Photosensitizers for Light-Driven Killing of Bacteria. Sci. Rep. 2018, 8, 15021.
- Liao, Y. Design and Applications of Metastable-State Photoacids. Acc. Chem. Res. 2017, 50, 1956–1964.
- Luo, Y.; Wang, C.; Peng, P.; Hossain, M.; Jiang, T.; Fu, W.; Liao, Y.; Su, M. Visible Light Mediated Killing of Multidrug-Resistant Bacteria Using Photoacids. J. Mater. Chem. B 2013, 1, 997–1001.
- Huang, T.-C.; Chen, C.-J.; Ding, S.-J.; Chen, C.-C. Antimicrobial Efficacy of Methylene Blue-Mediated Photodynamic Therapy on Titanium Alloy Surfaces In Vitro. Photodiagn. Photodyn. Ther. 2019, 25, 7–16.
- Yang, Y.; Goetzfried, M.A.; Hidaka, K.; You, M.; Tan, W.; Sugiyama, H.; Endo, M. Direct Visualization of Walking Motions of Photocontrolled Nanomachine on the DNA Nanostructure. Nano Lett. 2015, 15, 6672–6676.
- Zhuang, X.; Ma, X.; Xue, X.; Jiang, Q.; Song, L.; Dai, L.; Zhang, C.; Jin, S.; Yang, K.; Ding, B.; et al. A Photosensitizer-Loaded DNA Origami Nanosystem for Photodynamic Therapy. ACS Nano 2016, 10, 3486–3495.
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal Photodynamic Therapy. Microbiol. Res. 2008, 163, 1–12.
- Pereira Rosa, L. Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. J. Med. Microb. Diagn. 2014, 3, 1.
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294.
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736.
- Boca, S.C.; Four, M.; Bonne, A.; van der Sanden, B.; Astilean, S.; Baldeck, P.L.; Lemercier, G. An Ethylene-Glycol Decorated Ruthenium(II) Complex for Two-Photon Photodynamic Therapy. Chem. Commun. 2009, 30, 4590–4592.
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491.
- Liu, S.; Yuan, H.; Bai, H.; Zhang, P.; Lv, F.; Liu, L.; Dai, Z.; Bao, J.; Wang, S. Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics. J. Am. Chem. Soc. 2018, 140, 2284–2291.
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935.
- Kamanli, A.F.; Çetinel, G. Comparison of Pulse and Super Pulse Radiation Modes’ Singlet Oxygen Production Effect in Antimicrobial Photodynamic Therapy (AmPDT). Photodiagn. Photodyn Ther. 2020, 30, 101706.
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416.
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200.
- Liu, X.; Liu, J.; Chen, S.; Xie, Y.; Fan, Q.; Zhou, J.; Bao, J.; Wei, T.; Dai, Z. Dual-Path Modulation of Hydrogen Peroxide to Ameliorate Hypoxia for Enhancing Photodynamic/Starvation Synergistic Therapy. J. Mater. Chem. B 2020, 8, 9933–9942.
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-Assembled Single-Atom Nanozyme for Enhanced Photodynamic Therapy Treatment of Tumor. Nat. Commun. 2020, 11, 357.
- Nonell, S.; Ferreras, L.R.; Cañete, A.; Lemp, E.; Günther, G.; Pizarro, N.; Zanocco, A.L. Photophysics and Photochemistry of Naphthoxazinone Derivatives. J. Org. Chem. 2008, 73, 5371–5378.
- Lamarque, G.C.C.; Méndez, D.A.C.; Gutierrez, E.; Dionisio, E.J.; Machado, M.A.A.M.; Oliveira, T.M.; Rios, D.; Cruvinel, T. Could Chlorhexidine Be an Adequate Positive Control for Antimicrobial Photodynamic Therapy in-in Vitro Studies? Photodiagn. Photodyn. Ther. 2019, 25, 58–62.
- Pourhajibagher, M.; Bahador, A. Computational Biology Analysis of COVID-19 Receptor-Binding Domains: A Target Site for Indocyanine Green Through Antimicrobial Photodynamic Therapy. J. Lasers Med. Sci. 2020, 11, 433–441.
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294.
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736.
- Boca, S.C.; Four, M.; Bonne, A.; van der Sanden, B.; Astilean, S.; Baldeck, P.L.; Lemercier, G. An Ethylene-Glycol Decorated Ruthenium(II) Complex for Two-Photon Photodynamic Therapy. Chem. Commun. 2009, 30, 4590–4592.
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491.
- Liu, S.; Yuan, H.; Bai, H.; Zhang, P.; Lv, F.; Liu, L.; Dai, Z.; Bao, J.; Wang, S. Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics. J. Am. Chem. Soc. 2018, 140, 2284–2291.
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935.
- Kamanli, A.F.; Çetinel, G. Comparison of Pulse and Super Pulse Radiation Modes’ Singlet Oxygen Production Effect in Antimicrobial Photodynamic Therapy (AmPDT). Photodiagn. Photodyn Ther. 2020, 30, 101706.
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416.
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200.
- Liu, X.; Liu, J.; Chen, S.; Xie, Y.; Fan, Q.; Zhou, J.; Bao, J.; Wei, T.; Dai, Z. Dual-Path Modulation of Hydrogen Peroxide to Ameliorate Hypoxia for Enhancing Photodynamic/Starvation Synergistic Therapy. J. Mater. Chem. B 2020, 8, 9933–9942.
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-Assembled Single-Atom Nanozyme for Enhanced Photodynamic Therapy Treatment of Tumor. Nat. Commun. 2020, 11, 357.
- Nonell, S.; Ferreras, L.R.; Cañete, A.; Lemp, E.; Günther, G.; Pizarro, N.; Zanocco, A.L. Photophysics and Photochemistry of Naphthoxazinone Derivatives. J. Org. Chem. 2008, 73, 5371–5378.
- Lamarque, G.C.C.; Méndez, D.A.C.; Gutierrez, E.; Dionisio, E.J.; Machado, M.A.A.M.; Oliveira, T.M.; Rios, D.; Cruvinel, T. Could Chlorhexidine Be an Adequate Positive Control for Antimicrobial Photodynamic Therapy in-in Vitro Studies? Photodiagn. Photodyn. Ther. 2019, 25, 58–62.
- Pourhajibagher, M.; Bahador, A. Computational Biology Analysis of COVID-19 Receptor-Binding Domains: A Target Site for Indocyanine Green Through Antimicrobial Photodynamic Therapy. J. Lasers Med. Sci. 2020, 11, 433–441.
- Karolina Wojewoda; Martin Gillstedt; Jonatan Tovi; Louai Salah; Ann-Marie Wennberg Larkö; Alexandra Sjöholm; Carin Sandberg; Optimizing treatment of acne with photodynamic therapy (PDT) to achieve long-term remission and reduce side effects. A prospective randomized controlled trial. Journal of Photochemistry and Photobiology B: Biology 2021, 223, 112299, 10.1016/j.jphotobiol.2021.112299.
- Raphaëlle Youf; Max Müller; Ali Balasini; Franck Thétiot; Mareike Müller; Alizé Hascoët; Ulrich Jonas; Holger Schönherr; Gilles Lemercier; Tristan Montier; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995, 10.3390/pharmaceutics13121995.
This entry is offline, you can click here to edit this entry!
