Lignocellulosic biomass is the most abundant renewable feedstock to produce biofuels and biochemicals. Previous research has demonstrated the potential of bioleaching, with its superior capability of removing certain inorganic compounds compared to water leaching, to improve biomass quality for thermochemical conversion in biofuel production.
- lignocellulosic biomass
- bioleaching
- bioreactor
1. Introduction
Thermochemical conversion is one of the major platforms used to convert lignocellulosic biomass into bioenergy; however, it has faced issues because of the high ash content in most feedstocks, especially herbaceous biomass, e.g., wheat straw, corn stover, sorghum straw, etc. [1]. In the combustion or gasification of biomass without pretreatment, the inorganic ingredients in biomass could cause serious issues to reactors and the environment, such as slagging, fouling, agglomeration, corrosion, acid gas emission, etc. [1]. These issues not only reduce the heat transfer efficiency of the reactors, but also cause air pollution [2]. To improve the quality of lignocellulosic biomass as a potential fuel, a pretreatment known as leaching has been extensively studied and proven effective in removing inorganic ingredients in the biomass [3]. The most common pretreatment is water leaching, which simply washes the biomass with an adequate amount of water [4]. While this is effective in removing certain inorganic ingredients that are water-soluble, more effective methods are necessary to reduce elements such as Ca, Mg, Si, and S that tend to form insoluble compounds in the plant [5]. Bioleaching might be a solution to this issue, as it has been successfully applied to the mining and coal industries to extract metals (e.g., copper) from low grade ores and desulfurization, respectively [6]. Various bacterial and fungal species have been demonstrated for their bioleaching capability to treat a range of materials, from mining ores to municipal waste fly ash [7][8].
As a versatile industrial microbe, Aspergillus niger (A. niger) has been generally recognized as safe in standard industrial settings [9]. A. niger is the main producer of the citric acid widely used in food and pharmaceutical industries [10]. Furthermore, a variety of enzymes can be produced from A. niger, such as amylase, cellulase, xylanase, pectinase, etc. [11][12][13]. Recently this microbe has received extensive attention in hydrometallurgical and environmental investigations. Wu and Ting used A. niger to recover heavy metals from municipal solid waste (MSW) incinerator fly ash in both one-step and two-step leaching, and found that bioleaching achieved a higher recovery of Mn and Zn than chemical leaching [14]. Santhyia treated the spent refinery catalyst with A. niger to recover Al, Ni and Mo, in which the use of a buffer was found to be effective in stimulating oxalic acid secretion by this fungus, while also reducing the leaching time compared to the bioleaching process without the buffer addition [15]. Mulligan studied A. niger for the recovery of Cu and other metals from low-grade ores, and achieved 68% and 46% in the solubilization of Cu and Zn, respectively [16].
2. Pretreating Sorghum Straw
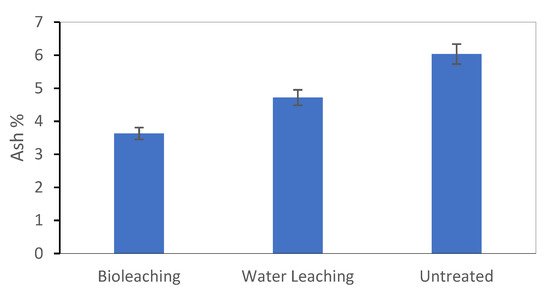
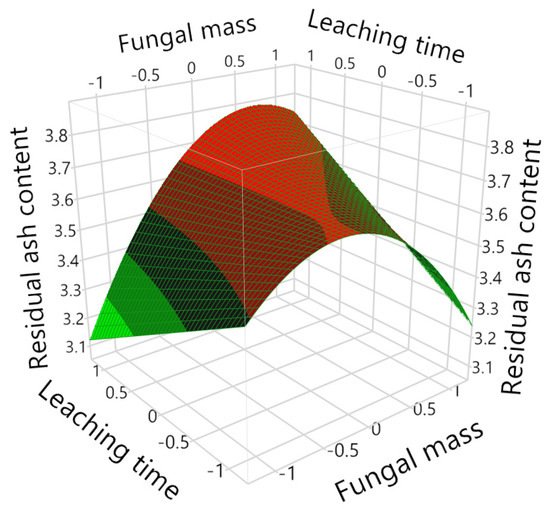
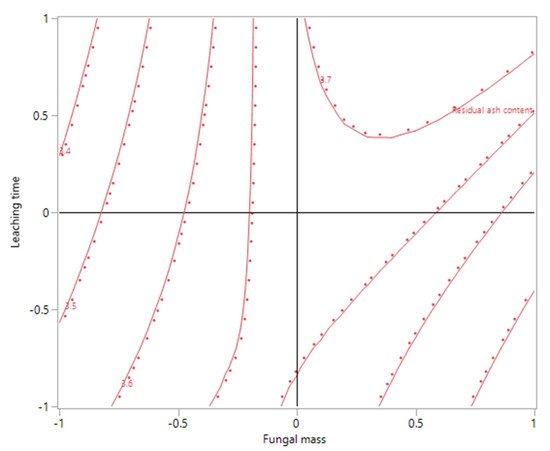
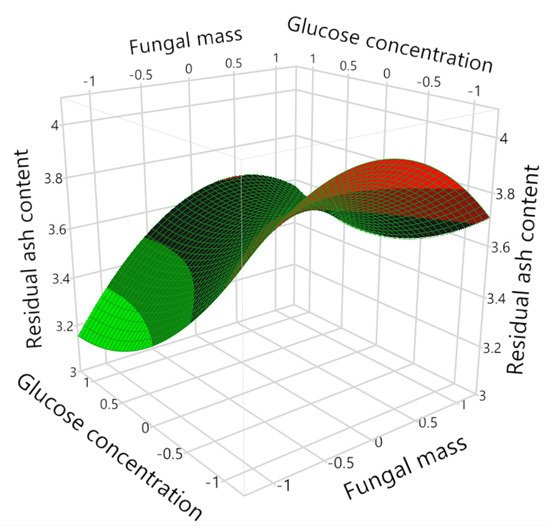
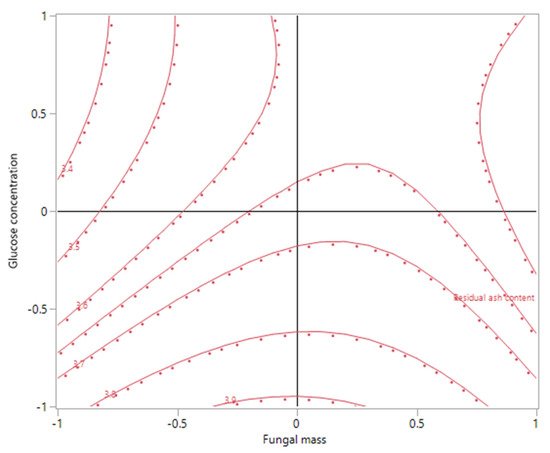
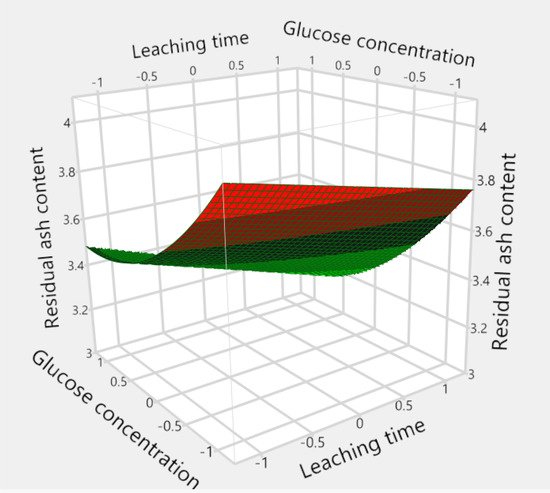
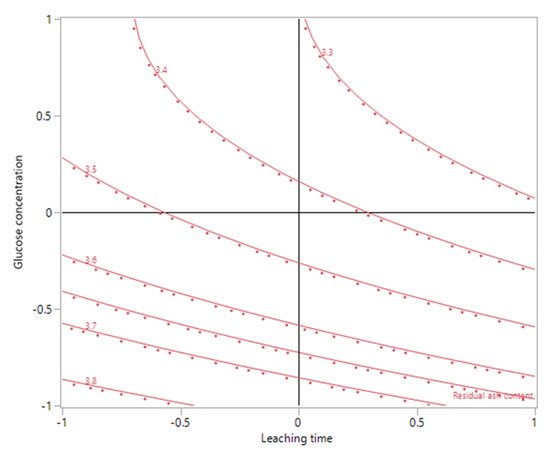
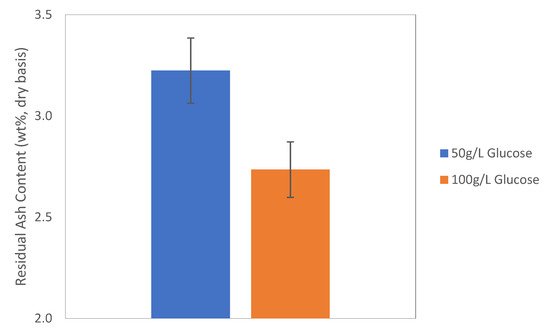
3. Ash Content in the Biomass
This entry is adapted from the peer-reviewed paper 10.3390/fermentation7040270
References
- Baxter, L.L.; Miles, T.R.; Miles, T.R., Jr.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1998, 54, 47–78.
- Vamvuka, D.; Zografos, D.; Alevizos, G. Control methods for mitigating biomass ash-related problems in fluidized beds. Bioresour. Technol. 2008, 99, 3534–3544.
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.; VanderGheynst, J.; Upadhyaya, S.; Jenkins, B. Influence of leaching pretreatment on fuel properties of biomass. Fuel Process. Technol. 2014, 128, 43–53.
- Carrillo, M.; Staggenborg, S.; Pineda, J. Washing sorghum biomass with water to improve its quality for combustion. Fuel 2014, 116, 427–431.
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279.
- Hocheng, H.; Su, C.; Jadhav, U.U. Bioleaching of metals from steel slag by Acidithiobacillus thiooxidans culture supernatant. Chemosphere 2014, 117, 652–657.
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552.
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A. Appl. Microbiol. Biotechnol. 2003, 63, 239–248.
- van Dijck, P.W.; Selten, G.C.; Hempenius, R.A. On the safety of a new generation of DSM Aspergillus niger enzyme production strains. Regul. Toxicol. Pharmacol. 2003, 38, 27–35.
- Ward, O.; Qin, W.; Dhanjoon, J.; Ye, J.; Singh, A. Physiology and biotechnology of Aspergillus. Adv. Appl. Microbiol. 2005, 58, 1–75.
- Sundarram, A.; Murthy, T.P.K. α-amylase production and applications: A review. J. Appl. Environ. Microbiol. 2014, 2, 166–175.
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.; van Dijck, P. On the safety of Aspergillus niger–a review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435.
- Guimaraes, N.d.A.; Sorgatto, M.; Peixoto-Nogueira, S.d.; Betini, J.; Zanoelo, F.; Marques, M.; Polizeli, M.; Giannesi, G. Bioprocess and biotechnology: Effect of xylanase from Aspergillus niger and Aspergillus flavus on pulp biobleaching and enzyme production using agroindustrial residues as substract. SpringerPlus 2013, 2, 1–7.
- Wu, H.; Ting, Y. Metal extraction from municipal solid waste (MSW) incinerator fly ash—Chemical leaching and fungal bioleaching. Enzym. Microb. Technol. 2006, 38, 839–847.
- Santhiya, D.; Ting, Y. Bioleaching of spent refinery processing catalyst using Aspergillus niger with high-yield oxalic acid. J. Biotechnol. 2005, 116, 171–184.
- Mulligan, C.N.; Kamali, M.; Gibbs, B.F. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J. Hazard. Mater. 2004, 110, 77–84.
- Yang, J.; Wang, Q.; Wang, Q.; Wu, T. Comparisons of one-step and two-step bioleaching for heavy metals removed from municipal solid waste incineration fly ash. Environ. Eng. Sci. 2008, 25, 783–789.
- Rasoulnia, P.; Mousavi, S. Maximization of organic acids production by Aspergillus niger in a bubble column bioreactor for V and Ni recovery enhancement from power plant residual ash in spent-medium bioleaching experiments. Bioresour. Technol. 2016, 216, 729–736.
- De Windt, L.; Devillers, P. Modeling the degradation of Portland cement pastes by biogenic organic acids. Cem. Concr. Res. 2010, 40, 1165–1174.
- Huang, K.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching of heavy metals by citric acid from fly ash generated in municipal waste incineration plants. J. Mater. Cycles Waste Manag. 2011, 13, 118–126.
- Umeda, J.; Kondoh, K. High-purity amorphous silica originated in rice husks via carboxylic acid leaching process. J. Mater. Sci. 2008, 43, 7084–7090.
- Zhang, N.; Wang, L.; Zhang, K.; Walker, T.; Thy, P.; Jenkins, B.; Zheng, Y. Pretreatment of lignocellulosic biomass using bioleaching to reduce inorganic elements. Fuel 2019, 246, 386–393.
