The vascular endothelium, the active inner layer of the blood vessel, releases a wide array of biologically active molecules acting in an autocrine or paracrine fashion, thereby controlling arterial structure and vasodilatory, thrombolytic, and vaso-protective functions. By controlling the change of the backbones of several cellular substrates, the peptidyl-prolyl cis-trans isomerase Pin1 acts as key fine-tuner and amplifier of multiple signaling pathways, thereby inducing several biological consequences, both in physiological and pathological conditions. Data from the literature indicate a prominent role of Pin1 in regulating vascular homeostasis.
1. Aging and the Vasculature: Focus on Endothelial Dysfunction
The vascular endothelium, the active inner layer of the blood vessel, releases a wide array of biologically active molecules acting in an autocrine or paracrine fashion, thereby controlling arterial structure and vasodilatory, thrombolytic, and vaso-protective functions. In particular, it regulates a number of biological functions, such as substrate exchange/transport, innate immunity, the regulation of vascular tone by balancing the production of vasodilators and vasoconstrictors, angiogenesis, and hemostasis by secreting antiplatelet and anticoagulant molecules (for a comprehensive review on the topic see [
1]). Endothelial dysfunction occurs during aging [
2], where an imbalance between vasodilator and vasoconstriction factors, a progressive reduction of nitric oxide (NO) bioavailability, as well as an increase in cyclooxygenase (COX)-derived vasoconstrictor factors have been reported [
2,
3]. In addition, a decreased expression and activity of endothelial NOS (eNOS) has been observed in older animals [
4]. Moreover, endothelial dysfunction represents a risk factor for the development of several human vascular diseases, such as atherosclerosis, hypertension and stroke, peripheral arterial disease, metabolic syndrome (obesity, insulin resistance), and diabetes. The molecular mechanisms underpinning endothelial dysfunction are rather complex. Indeed, multiple mechanisms (i.e. impaired vasodilation, oxidative stress, inflammation, cell injury/death, senescence) have been reported to be involved. Notably, among them, decreased NO bioavailability represents a key hallmark of endothelial dysfunction. Thus, the severity of endothelial dysfunction has been shown to have prognostic value for cardiovascular events [
5].
2. The Role of the cis-trans Isomerase Pin1 in Vascular Endothelium
Originally identified as a protein physically interacting with NIMA (never in mitosis A) mitotic kinase, Pin1 (protein interacting with NIMA-1) is a member of the evolutionarily conserved peptidyl-prolyl
cis-trans isomerase (PPIase) family that, unlike all other known prolyl isomerases, mediates the isomerization of phosphorylated serine- and threonine-proline (pSer/Thr-Pro) motifs, mediating the conformational change of cellular substrates [
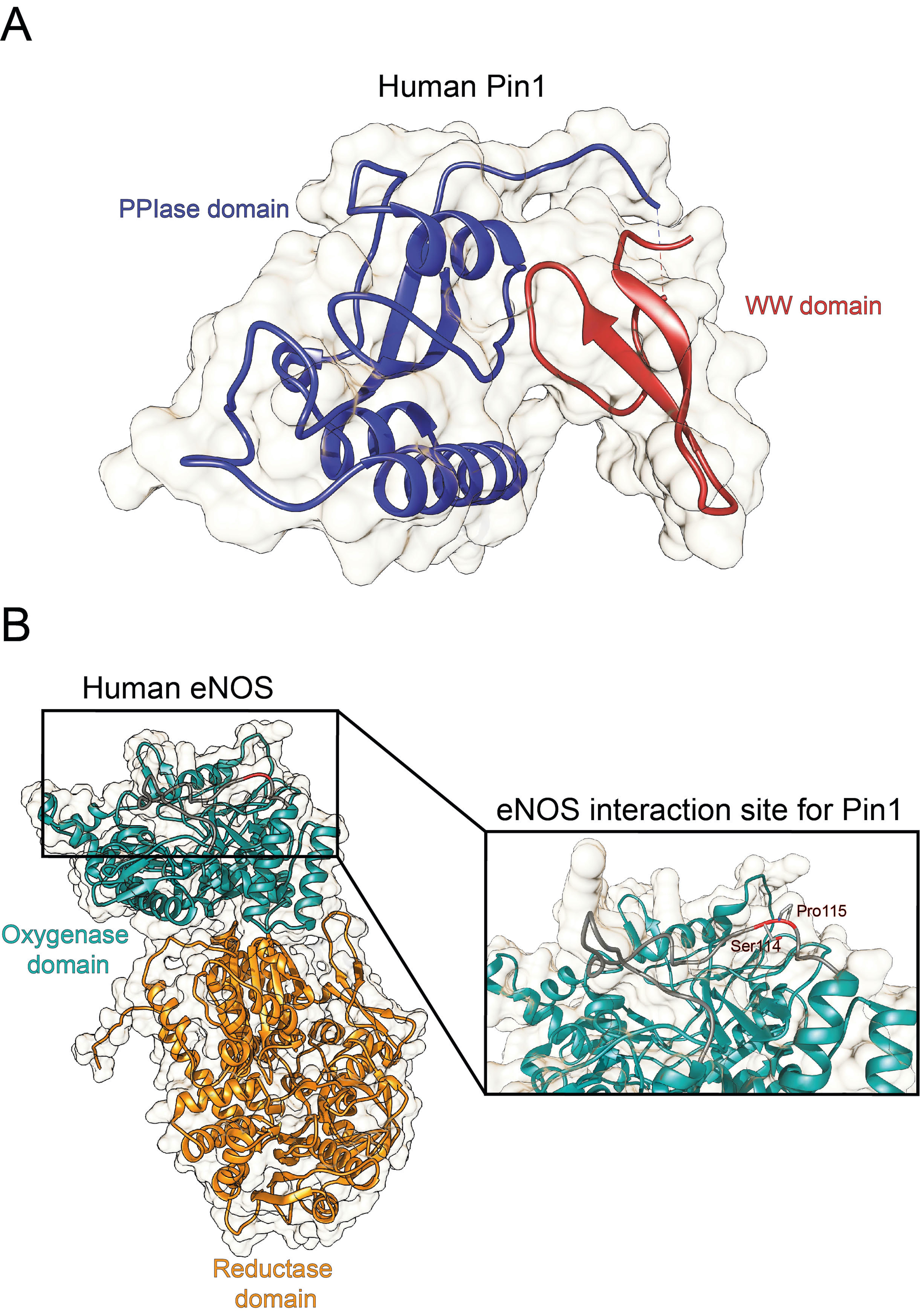
6]. From a structural point of view, human Pin1 contains an N-terminal WW protein interaction domain (residues 1–39) and a C-terminal PPIase domain (residues 50–163), connected by a flexible linker [
6] (
Figure 1A). Notably, Pin1-catalyzed phosphorylation-dependent conformational changes of its substrates have been reported to control many key regulators involved in numerous cellular processes (e.g., cell growth, cell cycle progression, cellular stress response, development, apoptosis, neuronal differentiation, and immune response) [
7]. In particular, it acts as a unique molecular timer regulating multiple targets at different steps of a given signaling pathway to synergistically orchestrate cellular responses [
7].
Notably, Pin1 deregulation has been associated with a number of pathological conditions, ranging from cancers [
8] to age-related and neurodegenerative diseases[
9]. Pin1 has been found to act as a critical driver of vascular cell proliferation, apoptosis, and inflammation, with implication in many cardiovascular diseases (e.g., atherosclerosis, coronary restenosis, and cardiac hypertrophy) [
10,
11,
12]. Notably, Pin1 has been reported to regulate the degradation of the inducible nitric oxide synthase (iNOS) in murine aortic endothelial cells upon induction by lipopolysaccharide and IFN-γ [
13], as well as to interact with eNOS in a phosphorylation-dependent manner (
Figure 1B
) , thereby suppressing eNOS activity, similar to the tonic suppression of eNOS activity by caveolin-1 [
14].
However, despite sparse evidence showing the implication of Pin1 in vascular homeostasis, little is known about Pin1 implication in cerebrovascular homeostasis, both in physiological and pathological contexts. As an example, Pin1 distribution and intracellular compartmentalization in the cerebrovascular system remains unknown. In the following sections, we will discuss evidence from the literature indicating the role of Pin1 in the regulation of vasculature homeostasis, by specifically dissecting its molecular interactions with key intracellular players, such as NOS, as well as the implication of Pin1 in vascular pathology. Moreover, we will discuss the hypothesis that Pin1 may serve a pivotal role in the function of vasculature, mainly focusing on its effects on the vascular endothelium. This latter, which is at direct contact with blood and affected by various vascular risk factors, represents a critical target and key player in vascular diseases.
Therefore, understanding the role of Pin1 in the vascular homeostasis and specifically, its interplay with NOS activity, is crucial in terms of finding a new possible therapeutic player and/or target in vascular pathologies, such as hypertension, diabetes, small and large vessel diseases, and vascular dementia.
3. Pin1 Implication in Vascular Diseases
Based on evidence indicating a key role of Pin1 in regulating NO production and vascular homeostasis, alteration of Pin1 levels and/or isomerase activity may be involved in the pathogenesis of vascular pathologies, such as hypertension and diabetes. In this regard, several studies described a prominent role of Pin1 in vascular changes associated to diabetes, where endothelial dysfunction represents an initial process in its vascular manifestations [
28]. Paneni et al. demonstrated that treatment of human aortic endothelial cells (HAECs) with high glucose concentration for 72 hours induced an increase in Pin1 mRNA and protein levels [
12]. In particular, methylation of Pin1 promoter, which typically represses gene transcription, was substantially decreased during hyperglycemia, suggesting that this epigenetic modification may contribute to Pin1 upregulation [
12]. Moreover, during hyperglycemia, Pin1 has been shown to interact with eNOS at Ser
116 phosphorylation site, thereby promoting eNOS interaction with caveolin-1, an important repressor of eNOS catalytic activity in the endothelium and subsequently reducing NO availability [
12]. Interestingly, diabetic Pin1
−/− mice were protected against mitochondrial oxidative stress, endothelial dysfunction, and vascular inflammation. In accordance with such evidence, pharmacological inhibition of Pin1 by juglone attenuated endothelial dysfunction, oxidative stress, as well as inflammatory processes, in streptozotocin-induced diabetic mice compared to vehicle-treated animals [
29]. Noteworthy, although these data suggest that Pin1 may hinder NO production by repressing eNOS activity during hyperglycemia, it is unknown whether such negative regulation may be ascribed either to a direct effect of Pin1 on eNOS activity or to the increased association of eNOS with caveolin-1 or to a combination of these two effects.
Moreover, high glucose levels not only triggered Pin1 expression, but also enhanced its enzymatic activity. Consistently, an observational study conducted in patients with type 2 diabetes mellitus showed that Pin1 expression levels and activity were increased in the peripheral blood monocytes of the subjects compared to those of age-matched healthy controls and correlated with glycemic markers (i.e., elevated fasting plasma glucose (FPG) and HbA1C (hemoglobin A1C) levels) [
30]. Experiments performed in type 2 diabetic (T2D) mice and in vascular smooth muscle cells exposed to T2D-ressembling conditions also showed that Pin1 levels were increased in hyperglycemic conditions [
30]. Finally, Zhang et al. confirmed the notion that high glucose levels up-regulate Pin1, by reporting a substantial elevation in circulatory Pin1 levels in diabetic mice compared to wild-type mice. Such increase was reversed with exposure to juglone [
31].
This entry is adapted from the peer-reviewed paper 10.3390/cells10123287

