Glucose is a six-carbon sugar, one of the most abundant in nature, and is the central element of human energy metabolism. Moreover, it is the main marker for the diagnosis of diabetes [
55,
56]. Glucose is an excellent model marker for developing paper-based POC devices due to its price, accessibility, ease of handling, lack of toxicity, relative chemical stability, high water solubility, and presence in relevant concentrations in various biological fluids [
56,
57]. Blood is the fluid of choice for the determination of biomarkers, and glucose is no exception. The reference range for fasting blood glucose in healthy individuals is 70–100 mg/dL [
58,
59]. Values below and above the reference range are relevant to health status, with low values being considered hypoglycemia and above being considered hyperglycemia. When values are greater than 125 mg/dL in two or more tests, it is possible to diagnose diabetes [
58,
59]. The intermediate stage with values of 100 to 125 mg/dL is defined as prediabetes and is a high-risk state for the development of T2DM [
58,
60]. The methods for blood glucose determination are well established, calibrated, and automatized, but sample collection is invasive, uncomfortable, and potentially painful. Most are enzyme-based, especially those based on glucose oxidase (GOD) with detection by colorimetry or electrochemistry [
56,
61]. The first generation of glucose biosensors is based on producing and detecting peroxide with oxygen as a cosubstrate (Equation (1)). This reaction involves the reduction of the flavin group (FAD) in the enzyme to generate the reduced form of the enzyme (FADH2) (Equation (2)) [
62,
63,
64].
These sensors have the disadvantage of being affected by electroactive interference, so some metabolites of interest and components of biological fluids can affect the selectivity of this type of sensor. Additionally, they can give erroneous readings due to fluctuations in oxygen availability [
62,
63,
64]. The second generation of glucose biosensors was achieved by replacing oxygen with a synthetic electron acceptor, acting as a mediator transporting electrons. Examples of these mediators are ferrocene-derived compounds, conductive organic salts, quinone compounds, and transition-metal complexes. In the third generation of glucose biosensors, electrons are directly transferred between the enzyme and the electrode without mediators. This type of biosensor has allowed the generation of devices for continuous in vivo glucose monitoring [
62,
63,
64]. The fourth generation of glucose biosensors comprises sensors based on metal nanostructures where glucose oxidation occurs directly on the electrode surface and does not require enzymes [
65,
66]. A more detailed analysis of non-enzymatic sensors, especially those based on electrochemistry for glucose determination, can be found in the review by Professor Wang et al. [
67]. Despite the undeniable progress that fourth-generation electrochemical devices represent, it is important to note that the use of nanomaterials for the fabrication of non-enzymatic devices increases the cost and complexity of the manufacturing processes, hindering their development and implementation in countries with limited resources. In contrast, enzyme-based devices, specifically those employing colorimetric detection, have attracted attention for detecting glucose in biological fluids due to their low development cost and versatility, making them of particular interest to countries with limited resources and will be the focus of the following discussion.
Colorimetry is the most reported technique for determining glucose in clinical samples, mainly through the bienzymatic system consisting of glucose oxidase (GOD) and horseradish peroxidase (HRP) coupled to chromogens. The reaction catalyzed by glucose oxidase results in gluconic acid and hydrogen peroxide production. Peroxidase catalyzes the reaction of the hydrogen peroxide with the chromogen(s) to generate the color change. The two most commonly used HRP chromogenic substrates are 4-amino antipyrine (4-AAP) and 3,3’,5,5’-tetramethylbenzidine (TMB) [
55,
57,
68,
69]. Examples of the widespread application of the bienzymatic system GOD/HRP are shown in
Table 1. It is vital to choose the right chromogen during the design of the paper-based assay platform to achieve suitable selectivity and specificity values for clinical application. There are several reports of using 4-AAP as a chromogen with detection limits relevant not only for the glucose determination in blood [
4,
45,
70,
71,
72,
73] but also in other biological fluids with low concentrations such as tears [
74], urine [
73], and saliva [
75,
76]. Other systems, such as GOD-HRP-TMB [
77] and GOD-HRP-o-dianisidine [
78], have shown promising results for detecting glucose in sweat.
Table 1. Summary of reported enzymatic paper-based platforms in representative references.
| Substrate |
System |
Sample |
Detection |
LOD (mg/dL) |
References |
| Wax printing on Whatman chromatography paper 595 |
GOD/HRP/
4-AAP/HBA |
Tears |
Smartphone camera |
NM |
[74] |
| Whatman filter paper No. 1 with lamination film |
GOD/BP |
Saliva |
Smartphone camera |
24.6 |
[75] |
| Wax printing on qualitative filter paper and Schirmer strips |
GOD/Au(I) complex (AuC2C6H4OMe)2 (Ph2P(C6H4)3PPh2) |
Simulated tear fluid and blood |
Bifurcated optical fiber system |
16.2 (plasma)
1.4 (tear) |
[79] |
| Whatman cellulose filter paper No. 1 treated with CH |
GOD/HRP/
EDC/o-PD |
Urine |
Smartphone camera |
18.0 |
[80] |
| Whatman filter paper No. 40 stamped with paraffin and treated with CH |
GOD/HRP/
TBHBA/4-AAP |
Artificial and human saliva |
Naked eye |
0.8 |
[76] |
| High-purity cellulose membranes |
GOD/HRP/TMB |
Urine |
Digital camera |
8.1 |
[69] |
| Whatman filter paper No. 1 with lamination film |
GOD/BP |
Saliva |
Handheld optical biosensor |
32.0 |
[81] |
| Wax printing on Whatman filter paper No. 1 |
GOD/HRP/
KI or TMB |
Plasma |
Smartphone camera |
27.0 (KI)
0.9 (TMB) |
[82] |
| Wax printing on Whatman filter paper No. 1 treated with CH |
GOD/HRP/
4-AAP/HBA |
Blood |
Scanner |
NM |
[70] |
| Whatman qualitative paper No.1 treated with PB |
GOD |
Serum |
Distance-based measurements |
19.8 |
[83] |
| Nitrocellulose membranes |
GOD/HRP/4-AAP/COL/MADB |
Serum |
Chemidoc imaging system |
0.2 |
[71] |
| Wax printing on Whatman No. 1 cellulose chromatography paper treated with BSA |
GOD/HRP/
4-AAP/DHBS |
Serum |
Scanner |
5.4 |
[4] |
| Whatman qualitative filter paper No. 1 coated with a UV-curable resin |
GOD/HRP/
MAOS/4-AAP |
Serum |
Smartphone camera |
5.4 |
[72] |
| Wax printing in Whatman No. 1 chromatography filter paper treated with CH |
GOD/HRP/TMB |
Blood |
Smartphone-based optical platform |
5.0 |
[84] |
| Whatman filter paper No. 3 treated with OTS and MTS |
GOD/HRP/
phenol/4-AAP |
Plasma |
Portable scanner |
15.1 |
[45] |
| Wax printing in Whatman No. 1 qualitative filter paper loaded with ZnNR |
GOD/
4-AAP/
DHBS |
Serum and urine |
Smartphone camera |
0.05 |
[73] |
| Whatman filter paper No. 41 treated with BSA-Tween |
GOD/HRP/TMB |
Sweat |
Scanner and Smartphone camera |
0.18 |
[77] |
In their 2017 report, Kang et al. reported using a paper platform made of cellulose filter paper to determine glucose in tears by colorimetry [
74]. Due to the low glucose concentration in tears, it is crucial to have a preconcentration step before the determination. The designed strip makes direct sampling possible due to its biocompatibility, and the printed wax barriers keep the reaction zone isolated from the sampling zone [
74]. This study demonstrated the detection of glucose in clinically relevant ranges. However, although they report that the color change allows differentiation between diabetic and normoglycemic patient samples, both with the naked eye and by optical density, they did not evaluate this quantitatively. Therefore, they did not report the detection limit, sensitivity, or specificity. In 2018, another group reported the preparation of two devices, a µPAD, and a Schirmer strip, according to the methodology reported by Kang et al. [
74] but with the use of a gold complex encapsulated in a carbopol gel to detect without chromogens [
79]. The µPAD was evaluated for the determination of blood glucose, and good performance for glucose selectivity and high reproducibility was observed, showing a strong linear correlation with the values obtained with a commercial glucometer [
79]. A study with a larger sample size would allow evaluating its potential to discriminate diabetic patients based on its sensitivity and specificity. The Schirmer strip treated with the gold complex was evaluated in simulated tear fluid, where a linear response between luminescence intensity and glucose concentration was observed [
79]. Further studies will be of great relevance in demonstrating such platforms' application with real patient samples and their clinical validation comparing tear samples from diabetic and normoglycemic patients.
The GOD/ HRP system coupled to potassium iodide (KI) or TMB was employed for blood glucose detection, the POC platform was generated with the wax printing method using Whatman No. 1 paper as support [
82]. In order to diminish the effect of ambient light on color detection, a stand with controlled illumination was designed to place the smartphone [
82]. One of the main factors affecting microfluidic platforms is the sample volume variation, which is a challenge that needs to be overcome to achieve the commercial application of a POC platform since it would require users to introduce a standard amount of sample. This paper made a comparison between a volume-independent platform (VI-µPAD) and a conventional platform (C-µPAD). In the conventional platform, it is observed that the color intensity increases with higher sample volume, even though the glucose concentration remains constant [
82]. In the proposed VI-µPAD platform, the sample comes in direct contact with the enzymes and chromogen, and it is the colored product that travels to the detection zone, allowing a homogeneous and uniform color intensity. Moreover, this color is more related to the glucose concentration than the sample volume. In addition, the use of TMB instead of KI for detection allows the detection limit to be significantly lower, without sacrificing its broad linear range (0–22 mM), which would allow its use for real samples with clinically relevant values [
82]. In 2020, a similar wax-printed, chitosan-treated paper system sealed with lamination film to configure the µPAD was reported [
70]. This µPAD employs a system based on peroxide generation by GOD and lactate oxidase (LOD) enzymes and its subsequent colorimetric detection by the HRP/4-AAP/DHBS system, and it includes a separation membrane to allow its use on blood samples directly without the need for pretreatment, and the color change detection was performed by capturing the images with a scanner. This system showed the ability to accurately detect glucose in serum and whole blood with high linearity and recovery rates in the range of 90–110%. The same research group reported a proof-of-concept of a similar platform for the simultaneous determination of glucose and lactate [
4]. This system showed good selectivity; no significant color generation was observed when using interfering solutions (fructose, lactose, sucrose, NaCl, MgCl
2, CaCl
2, L-cysteine, and uric acid) as samples. The high selectivity for the biomarkers, conferred by the catalytic properties of GOD and LOD, would allow the use of this platform with serum samples [
4]. In this system, it was possible to obtain a color change distinguishable to the naked eye for both analytes, and in addition, this µPAD has self-calibration capabilities. Furthermore, in the future, by incorporating a smartphone, it would be possible to move from a semi-quantitative to a quantitative detection.
The GOD-catalyzed reaction of glucose to generate gluconic acid causes a pH change in the medium that can be detected using a pH indicator. Examples of such systems using bromocresol purple as an indicator have been reported, and these systems were able to determine glucose in saliva with high sensitivity and accuracy [
75,
81,
85]. In their
2015 study, this research group reported a proof-of-concept using methyl red as a pH indicator and an office scanner as the device to acquire the color signal. However, despite showing potential in clinical ranges, this platform showed a high LOD of 22.2 mg/dL and was strongly affected by interferents commonly present in the samples, such as lactic and ascorbic acids [
85]. Their 2017 report [
75] employed purple bromocresol as a pH indicator and a smartphone as a platform for color data acquisition. While in their subsequent work in 2019 [
81], they reported using a standalone electronic meter, which avoids variability due to ambient light conditions. This device was validated with clinical saliva samples, and its performance was compared against blood glucose values measured with a conventional glucometer. In addition, a high correlation was observed between blood glucose and saliva glucose values of diabetic patients [
81]. Other methods and approaches that have been evaluated for the generation of platforms of this type are substrate treatment with UV resins [
72], organosilanes [
45], and the coupling of GOD and 4-AAP with nanoparticles [
73]. The generation of analytical platforms that do not require an electronic readout device, i.e., naked eye determinations, has been explored [
86]. In a 2018 report, detection by the naked eye was evaluated on a paper platform with hydrophobic lanes generated by patterning with paraffin [
76]. A chitosan treatment on the substrate improved the distribution of the reagents, generating a more homogeneous color reaction and increasing the material’s biocompatibility with the GOD/HRP bienzymatic system. In addition, this system was evaluated on saliva samples where it was shown to be accurate with recovery rates of 92 to 114% and low operator variation [
76]. The color change obtained can be used to construct a semi-quantitative scale to determine glucose levels with the naked eye, similarly to urine test strips. The specific design features of a POC platform should be evaluated based on the biomarker and the intended use. Systems that generate qualitative or semi-quantitative results can monitor already diagnosed patients or screen patients with high-risk profiles, which can be enhanced with the generation of simple, portable, and even readable devices. In contrast, more accurate systems that generate quantitative results may be reserved for diagnosis and use in clinical settings where portability can be allowed to be reduced to some extent to accommodate more sophisticated reading methods. Colorimetry-based POC devices have been shown to be a viable alternative for method development for either of these approaches.
Despite the development of new technologies for glucose detection, interest in the use of colorimetry for glucose determination has not diminished in recent years [
87]. This interest is evidenced by the steady increase in the number of publications on the subject in the last ten years, presented in
Figure 1.
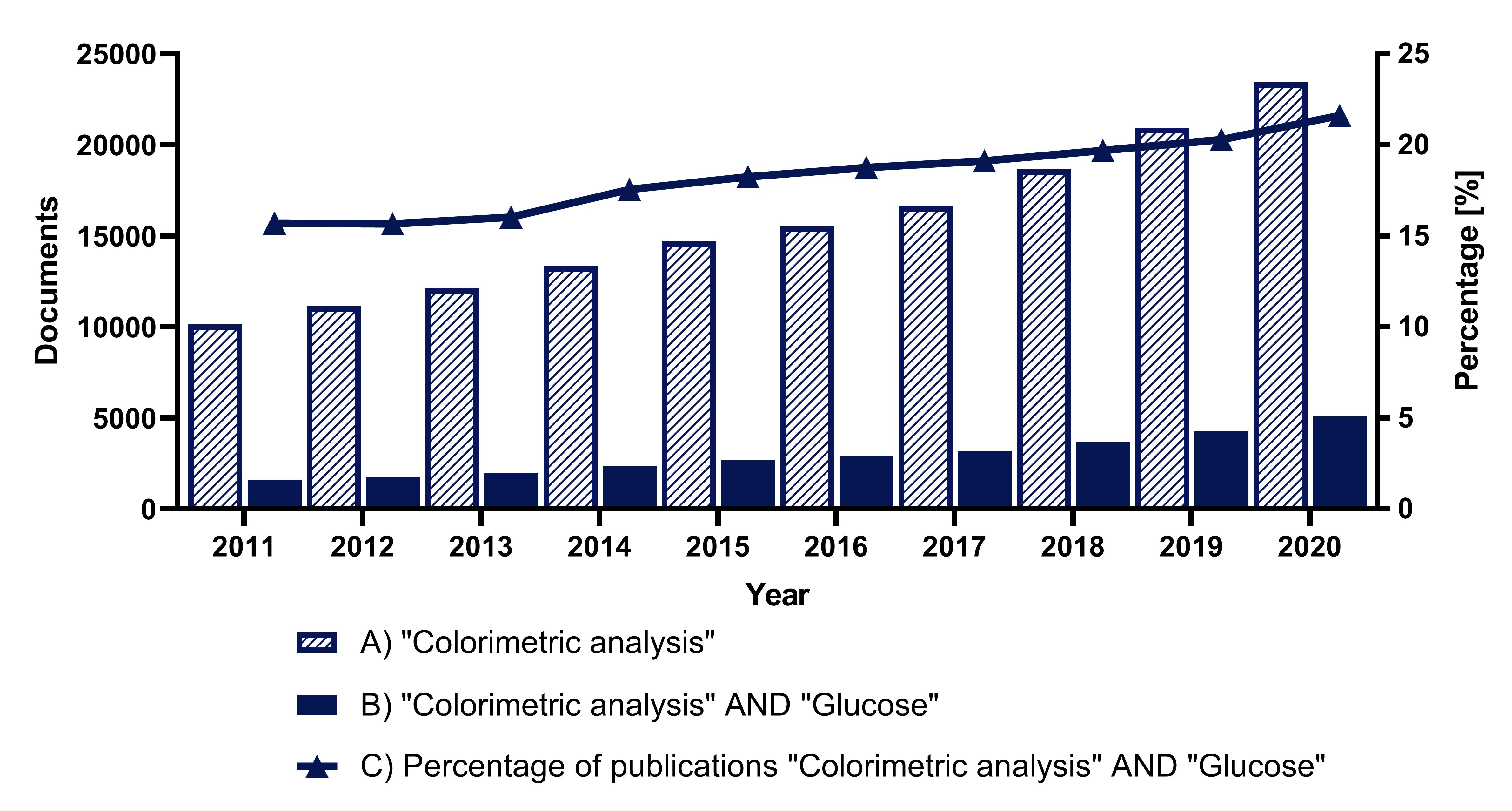 Figure 1.
Figure 1. Analysis of the number of documents in Scopus over the last ten years: (
A) using the search term “Colorimetric analysis”, (
B) using the search terms “Colorimetric analysis” AND “Glucose”, (
C) percentage of papers found using the term “Glucose” in the category “Colorimetric analysis”.
Since the recommendation made in 2009 by the International Expert Committee regarding glycated hemoglobin (HbA1c) as a long-term glycemic marker, this biomarker has been incorporated into worldwide clinical guidelines as a fundamental test for screening, monitoring, and diagnosis of T2DM [
88,
89,
90]. One of the limitations of this biomarker is that the test must be carried out by a standardized and certified method to ensure the validity of the results. This standardization has been achieved in the United States and other parts of the world thanks to the National Glycohemoglobin Standardization Program (NGSP) and the Diabetes Control and Complications Trial (DCCT) assay [
91,
92]. Although POC devices for determining HbA1c are already on the market, these analyzers require a specific setting that limits their use outside clinical facilities, and their cost is not accessible, so the balance between portability, cost, and accuracy has not yet been achieved [
93]. Several reports have evaluated the performance of POC analyzers compared to tests routinely employed in clinical laboratories. For example, in a meta-analysis published in 2017 [
94], thirteen devices were evaluated, A1cgear, A1cNow, Afinion, B-analyst, Clover, Cobas b101, DCA 2000/Vantage, HemoCue, Innovastar, Nycocard, Quo-Lab, Quo-Test, and SDA1cCare. Nine of these devices showed a negative bias and large standard deviations, negatively affecting disease management [
94]. In another study, the AfinionTM AS100 (Axis-Shield, Oslo Norway) and DCA VantageTM (Siemens Healthcare Diagnostics, Tarrytown, NY, US) analyzers were evaluated in comparison to conventional HPLC, both showing a good correlation with the conventional method [
93]. However, both analyzers reported significantly lower values [
93]. Subsequent studies have shown that analyzers have improved their performance [
95,
96,
97]. However, some still present differences compared to conventional methods and should be used with caution in patients with renal failure. Moreover, the fact that they still require to be implemented in controlled settings prevents the development of a portable and accessible POC with its full potential [
95,
96,
97]. Electrochemical microfluidic devices for HbA1c determination have also been reported [
44,
98,
99]. Specifically, electrochemical impedance spectroscopy (EIS) has attracted attention for being a non-destructive and very sensitive biosensing technique. A three-dimensional paper-based device with EIS detection for the simultaneous determination of total hemoglobin and HbA1c was reported, showing high sensitivity for both analytes in ranges of clinical interest and a detection limit of 0.21% for HbA1c [
44]. A nanobiosensor with a three-dimensional gold structure has also been documented to determine HbA1c in blood. Although it possesses the desirable characteristics of high sensitivity (269.2 mA/cm
2) and low detection limit (0.0068 mg/dL), the concentration of HbA1c in blood is above the linear range of the biosensor, requiring sample dilution [
99]. Undoubtedly, the use of nanomaterials to develop paper-based devices with electrochemical detection has driven the advancement of HbA1c determination. Thus, this fourth generation of biosensors represents an undeniable potential in the area of POC for diabetes screening and diagnosis.
Glycosylated albumin (GA) is another emerging biomarker for the screening and diagnosis of diabetes [
100,
101]. The most exploited methods for isolating and quantifying GA at a clinical scale are affinity chromatography and enzymatic assays. One of its differential characteristics is that it has an intermediate detection range (2–3 weeks), and in some populations, it has shown a better performance than HbA1c for monitoring glycemic levels [
101,
102,
103,
104]. Despite the relevance of GA as a glycemic biomarker, there are still no commercially available POC devices for its determination. Nevertheless, it is expected that advances will soon emerge due to its potential and the high incidence of T2DM [
105]. The development of microfluidic platforms for GA determination has benefited from nanomaterials that eliminate the need for the use of chromogens and enzymes. This technology has been used to develop a dipstick for GA determination achieving a detection limit of 6.59 μM in buffer and 8.7 μM in bovine serum [
106]. The use of enzymatic processes has proven to be an area of interest for developing analytical platforms for disease monitoring and diagnosis. Currently, commercial kits are available to determine glycemic markers such as glucose, GA, and fructosamines (FAs). It is possible to use a clinically validated enzymatic method as a basis for developing POC devices, optimizing them for portability, and avoiding the need for a clinical laboratory [
107]. A 2017 paper [
107] reported an electrochemical sensor based on the coupling of an enzymatic method with a screen-printed carbon electrode for GA detection that could be used to develop a POC platform. This same research group reported the development of an enzyme-based electrochemical sensor, but this time they used an interdigitated electrode that allowed them to improve the sensitivity (2.8 nA/µM) and detection limit (1.2 µM) concerning their previous work [
108]. Paper-based platforms can exploit their capabilities to generate multiplex assays, as in the case of a paper published in 2020 [
71], which reports the simultaneous determination of hemoglobin, GA, and glucose on a paper-based platform with colorimetric detection. This platform showed detection limits of 0.23 mg/dL, 49.16 ng/mL, and 8.36 μg/mL for glucose, albumin, and GA, respectively [
71].
FAs are a by-product of serum protein glycosylation that can serve as a marker of glycemic level [
109,
110,
111]. This marker, like GA, represents an intermediate monitoring marker (2–3 weeks). Although commercial kits for FAs determination are available in some countries, their use in the clinic is limited [
112,
113]. The development of POC devices for FAs determination has not generated as much interest as other glycemic markers mentioned above. The development of a paper-based microfluidic platform using a wax-dipping process has been reported [
111]. This platform allowed the determination of FAs corrected for variation in serum albumin by colorimetry using whole blood as a sample, with a membrane attached to the device for plasma separation [
111]. Despite the advances in these biomarkers, glucose continues to be one of the most studied as a model molecule for the development of sensors and POC devices. In addition, the devices for its determination are among the most advanced not only in the management of diabetes but in general in the diagnostic area. It is also one of the only biomarkers with continuous monitoring devices clinically validated and available on the market [
105].
As the burden of diabetes grows worldwide and is especially critical for resource-limited countries, there is a growing interest in cost-effective alternatives for the screening and early diagnosis of T2DM [
55]. For a novel platform to be accepted, the users’ point of view must be considered. The test should be easy to use, affordable, painless, and non-invasive, it should not require expensive or hard-to-maintain equipment, and it should present the results in a way that is to interpret [
57]. There is currently a growing interest in developing novel, sensitive, accurate, rapid, and cost-effective methods for glucose detection. Paper-based POCs are an excellent alternative for conventional lab testing in T2DM because, in addition to meeting all these requirements, they have advantages such as portability and minimal sample consumption [
55]. In addition, by using non-conventional sample fluids such as tears, sweat, or saliva, it would be possible to develop non-invasive platforms, which offer a competitive advantage in the market against traditional tests. Paper-based platforms have proven to be excellent alternatives for developing POC tests, and as mentioned in this review, their use in conjunction with colorimetric analysis has obvious advantages and benefits. However, one of their areas of opportunity is the limit of detection, which may prevent their application in non-conventional fluids. To overcome this challenge, other detection approaches have been analyzed, such as detection by electrochemical methods [
114,
115], distance-based [
83,
116], luminescence [
79,
117], fluorescence [
79,
118], calorimetry [
119,
120], and mass spectra [
121,
122]. Most of the paper-based POCs reported in
Table 1 of this review have reported stability under refrigeration (4 °C) [
4,
69,
73,
76,
79,
80,
84,
123]. However, it would be better to ensure that the devices retain acceptable stability and low variability at different environmental conditions for mass implementation in screening programs.
 Figure 1. Analysis of the number of documents in Scopus over the last ten years: (A) using the search term “Colorimetric analysis”, (B) using the search terms “Colorimetric analysis” AND “Glucose”, (C) percentage of papers found using the term “Glucose” in the category “Colorimetric analysis”.
Figure 1. Analysis of the number of documents in Scopus over the last ten years: (A) using the search term “Colorimetric analysis”, (B) using the search terms “Colorimetric analysis” AND “Glucose”, (C) percentage of papers found using the term “Glucose” in the category “Colorimetric analysis”.