Anion exchange membrane fuel cells (AEMFC) are clean energy conversion devices that are an attractive alternative to the more common proton exchange membrane fuel cells (PEMFCs), because they present, among others, the advantage of not using noble metals like platinum as catalysts for the oxygen reduction reaction. The AEMs are the central element of many technologically relevant devices, first and foremost alkaline membrane fuel cells (FC). These fuel cells can significantly reduce the amount of noble metal catalysts for the oxygen reduction reaction (ORR) and may represent the future of FC development.
- AEMFCs
- carbon nanotubes
- LDH
- silica
- graphene
- MOF
- PVA
- PPO
- QCDs
1. 1D Materials
1.1. Titanate (TNTs) and Halloysite Nanotubes (HNTs)
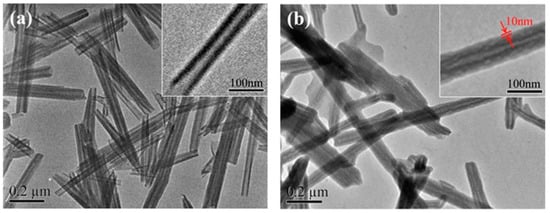
1.2. Carbon Nanotubes (CNTs)
-
SWCNT (Single-Walled Carbon NanoTubes) consist of a single graphitic sheet wound on itself.
- MWCNT (Multi-Walled Carbon NanoTubes) are formed by several sheets coaxially wound one on top of the other.
2. 2D Materials
2.1. Layered Double Hydroxides (LDHs)
2.1.1. Poly(vinyl alcohol) (PVA) and Chitosan (CS)

2.1.2. Polysulfone (PSU)
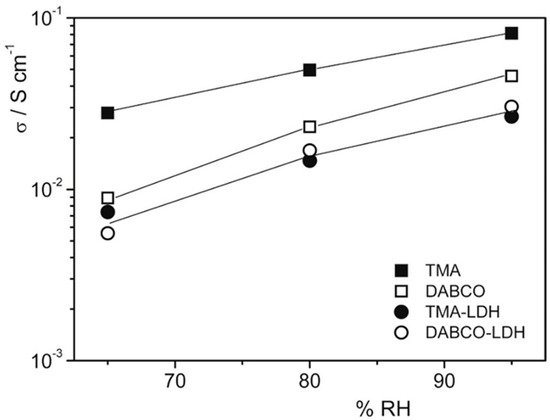
2.2. MXenes
3. 3D Materials
3.1. Silica and Silicates
3.1.1. Poly(vinylidene fluoride) (PVDF) and Poly(vinyl alcohol) (PVA)
3.1.2. Aromatic Polymers
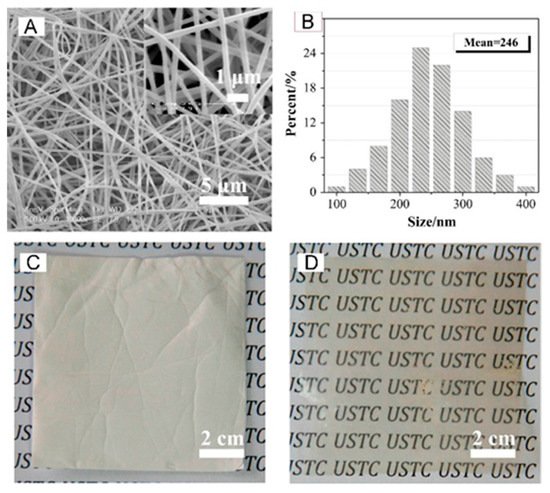
3.2. Metal Oxides and Derivatives
3.2.1. Aluminum Oxides
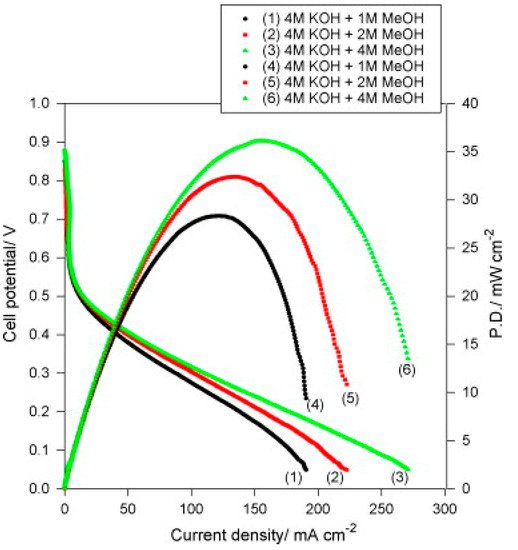
3.2.2. Titanium Dioxide and Titanates
This entry is adapted from the peer-reviewed paper 10.3390/polym13223887
References
- Abdullah, M.; Kamarudin, S. Titanium dioxide nanotubes (TNT) in energy and environmental applications: An overview. Renew. Sustain. Energy Rev. 2017, 76, 212–225.
- Premchand, Y.D.; Djenizian, T.; Vacandio, F.; Knauth, P. Fabrication of self-organized TiO2 nanotubes from columnar titanium thin films sputtered on semiconductor surfaces. Electrochem. Commun. 2006, 8, 1840–1844.
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334.
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163.
- Elumalai, V.; Sangeetha, D. Preparation of anion exchangeable titanate nanotubes and their effect on anion exchange membrane fuel cell. Mater. Des. 2018, 154, 63–72.
- Elumalai, V.; Sangeetha, D. Synergic effect of ionic liquid grafted titanate nanotubes on the performance of anion exchange membrane fuel cell. J. Power Sources 2018, 412, 586–596.
- Lu, Y.; Hu, Z.; Buregeya, I.; Pan, X.; Li, N.; Chen, S.; Liu, Y. Composite Anion Exchange Membranes from Quaternized Poly(arylene ether ketone) and Quaternized Titanate Nanotubes with Enhanced Ion Conductivity. Fuel Cells 2019, 19, 663–674.
- Shi, B.; Li, Y.; Zhang, H.; Wu, W.; Ding, R.; Dang, J.; Wang, J. Tuning the performance of anion exchange membranes by embedding multifunctional nanotubes into a polymer matrix. J. Membr. Sci. 2016, 498, 242–253.
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205.
- Zeng, L.; Zhao, T.; Li, Y. Synthesis and characterization of crosslinked poly (vinyl alcohol)/layered double hydroxide composite polymer membranes for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2012, 37, 18425–18432.
- He, X.Y.; Cao, L.; He, G.W.; Zhao, A.Q.; Mao, X.L.; Huang, T.; Li, Y.; Wu, H.; Sun, J.; Jiang, Z.Y. A highly conductive and robust anion conductor obtained via synergistic manipulation in intra- and inter-laminate of layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 10277–10285.
- Hu, Y.; Tsen, W.-C.; Chuang, F.-S.; Jang, S.-C.; Zhang, B.; Zheng, G.; Wen, S.; Liu, H.; Qin, C.; Gong, C. Glycine betaine intercalated layered double hydroxide modified quaternized chitosan/polyvinyl alcohol composite membranes for alkaline direct methanol fuel cells. Carbohydr. Polym. 2018, 213, 320–328.
- Zhao, S.; Tsen, W.-C.; Hu, F.; Zhong, F.; Liu, H.; Wen, S.; Zheng, G.; Qin, C.; Gong, C. Layered double hydroxide-coated silica nanospheres with 3D architecture-modified composite anion exchange membranes for fuel cell applications. J. Mater. Sci. 2019, 55, 2967–2983.
- Gong, C.; Zhao, S.; Tsen, W.-C.; Hu, F.; Zhong, F.; Zhang, B.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Hierarchical layered double hydroxide coated carbon nanotube modified quaternized chitosan/polyvinyl alcohol for alkaline direct methanol fuel cells. J. Power Sources 2019, 441, 227176.
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435.
- Li, C.; Sun, G.; Ren, S.; Liu, J.; Wang, Q.; Wu, Z.; Sun, H.; Jin, W. Casting Nafion–sulfonated organosilica nano-composite membranes used in direct methanol fuel cells. J. Membr. Sci. 2006, 272, 50–57.
- Adjemian, K.T.; Lee, S.J.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Silicon oxide Nafion composite membranes for proton-exchange membrane fuel cell operation at 80–140 °C. J. Electrochem. Soc. 2002, 149, A256–A261.
- Sgreccia, E.; Narducci, R.; Knauth, P.; Di Vona, M. Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review. Polymers 2021, 13, 1874.
- Zuo, X.; Yu, S.; Xu, X.; Xu, J.; Bao, R.; Yan, X. New PVDF organic–inorganic membranes: The effect of SiO2 nanoparticles content on the transport performance of anion-exchange membranes. J. Membr. Sci. 2009, 340, 206–213.
- Liu, G.; Tsen, W.-C.; Jang, S.-C.; Hu, F.; Zhong, F.; Zhang, B.; Wang, J.; Liu, H.; Wang, G.; Wen, S.; et al. Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells. J. Membr. Sci. 2020, 611, 118242.
- Wang, E.D.; Zhao, T.S.; Yang, W.W. Poly (vinyl alcohol)/3-(trimethylammonium) propyl-functionalized silica hybrid membranes for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 2183–2189.
- Yang, C.-C.; Chiu, S.-S.; Kuo, S.-C.; Liou, T.-H. Fabrication of anion-exchange composite membranes for alkaline direct methanol fuel cells. J. Power Sources 2012, 199, 37–45.
- Kumar, S.R.; Juan, C.-H.; Liao, G.-M.; Lin, J.-S.; Yang, C.-C.; Ma, W.-T.; You, J.-H.; Lue, S.J. Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells. Energies 2015, 9, 15.
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A cellulose nanocrystal-based composite electrolyte with superior dimensional stability for alkaline fuel cell membranes. J. Mater. Chem. A 2015, 3, 13350–13356.
- Pan, J.F.; He, Y.B.; Wu, L.; Jiang, C.X.; Wu, B.; Mondal, A.N.; Cheng, C.L.; Xu, T.W. Anion exchange membranes from hot-pressed electrospun QPPO-SiO2 hybrid nanofibers for acid recovery. J. Membr. Sci. 2015, 480, 115–121.
- Yang, C.C.; Chiu, S.J.; Chien, W.C.; Chiu, S.S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Sources 2010, 195, 2212–2219.
- Vinodh, R.; Sangeetha, D. Comparative study of composite membranes from nano-metal-oxide-incorporated polymer electrolytes for direct methanol alkaline membrane fuel cells. J. Appl. Polym. Sci. 2012, 128, 1930–1938.
- Nonjola, P.T.; Mathe, M.K.; Modibedi, R.M. Chemical modification of polysulfone: Composite anionic exchange membrane with TiO2 nano-particles. Int. J. Hydrogen Energy 2013, 38, 5115–5121.
- Xie, F.; Gao, X.; Hao, J.; Yu, H.; Shao, Z.; Yi, B. Preparation and properties of amorphous TiO2 modified anion exchange membrane by impregnation-hydrolysis method. React. Funct. Polym. 2019, 144, 104348.
- Msomi, P.; Nonjola, P.; Ndungu, P.; Ramontja, J. Poly (2, 6-dimethyl-1, 4-phenylene)/polysulfone anion exchange membrane blended with TiO2 with improved water uptake for alkaline fuel cell application. Int. J. Hydrogen Energy 2020, 45, 29465–29476.
- Derbali, Z.; Fahs, A.; Chailan, J.-F.; Ferrari, I.; Di Vona, M.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189.
- Chu, Y.; Chen, Y.; Chen, N.; Wang, F.; Zhu, H. A new method for improving the ion conductivity of anion exchange membranes by using TiO2 nanoparticles coated with ionic liquid. RSC Adv. 2016, 6, 96768–96777.
- Chen, Y.; Li, Z.; Chen, N.; Li, R.; Zhang, Y.; Li, K.; Wang, F.; Zhu, H. A new method for improving the conductivity of alkaline membrane by incorporating TiO2-ionic liquid composite particles. Electrochim. Acta 2017, 255, 335–346.
