“Post-acute sequelae of SARS-CoV-2 infection” (PASC), otherwise referred to as “long COVID” or “long-haul COVID”, refers to persistent and prolonged effects after acute COVID-19 and describes the persistence of symptoms or development of sequelae beyond 3 weeks from the onset of acute symptoms of SARS-CoV-2 infection. Symptoms commonly experienced by PASC patients include fatigue, palpitations, chest pain, dyspnea, reduced exercise tolerance, and “brain fog”. Additionally, symptoms of orthostatic intolerance and syncope suggest the involvement of the autonomic nervous system.
1. Post-Acute Sequelae of COVID-19
COVID-19 survivors have been shown to experience a constellation of symptoms such as fatigue, dyspnea or breathlessness [8,15], palpitations [16,17], brain fog, lack of concentration [18,19], sleep disturbances (i.e., insomnia) [20], headache [17,21,22,23,24,25], orthostatic intolerance [25], anxiety and post-traumatic stress disorder [20,23,26,27,28], chest pain, joint pain [8], sore throat [17], and hair loss [15] persisting >4 weeks after recovery. These clinical sequelae share similarities with post-acute symptoms reported after severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) epidemics, caused by previous coronaviruses [14]. Such heterogeneous postmorbid clinical presentation, with a plethora of specific signs and symptoms often being interpreted as non-physiologic or related to mental-health-related conditions, lends itself to the potential marginalization of patients [28].
Fatigue is by far the most commonly reported symptom among subjects with previous COVID-19 infection. Severe fatigue is more frequently reported by female and younger patients [32], and was associated with a high prevalence of anxiety [33] and post-traumatic stress disorder. Notably, fatigue described by PASC patients shows some distinct features from chronic fatigue syndrome (CFS). Studies on CFS patients showed impaired cerebral blood flow [34] and reduced heart rate variability [35,36], although these findings could not be confirmed in PASC patients [33]. Furthermore, less than 50% of patients with PASC-related fatigue met the diagnostic criteria for CFS [37]. This suggests that PASC is not just a COVID-19-related manifestation of CFS but a specific pathophysiological entity with a specific CVAD phenotype.
2. COVID-19 Infection and Dysautonomia
A growing body of evidence links PASC to CVAD, with a number of reports suggesting the possibility of previously infected COVID-19 patients experiencing symptoms suggestive of POTS [
10,
13,
16,
43,
44,
45,
46]. This is also consistent with previous evidence hinting at the presence of CVAD in SARS survivors during the 2003 epidemic [
47].
It should be emphasized that POTS is a rare medical condition, and the onset of tachycardia following SARS-CoV-2 or any other viral infection can be more frequently explained as a physiological response of a perfectly intact autonomic nervous system. It has been recently suggested that post-COVID-19 tachycardia syndrome would represent a distinct disease entity, different from classical POTS [
48].
Possible causes of tachycardia in COVID-19 survivors may include systemic conditions, deconditioning, anemia, hypoxia, anxiety, persisting fever, lung or cardiac disease, including sinus node dysfunction, myocarditis, and heart failure. Accordingly, comprehensive clinical workup and the appropriate use of cardiovascular and autonomic tests are mandatory to ensure accurate diagnosis.
3. Postural Orthostatic Tachycardia Syndrome after COVID-19
Beyond appropriate sinus tachycardia, which comprises both physiological and pathologic causes of increased heart rate, a possible explanation for palpitations and fatigue in COVID-19 survivors is POTS, which is a dysautonomia characterized by an excess in heart rate increase on standing and orthostatic intolerance [
49]; it is more frequent in females (80% of cases), and the young (especially 15–45 years old). POTS is misdiagnosed in up to 75% of patients and viral infection is recognized as a trigger in up to 41% of cases [
63].
In March 2021, the American Autonomic Society (AAS) issued a statement concerning the clinical overlap of PASC with POTS [
4]. The AAS stated that most PASC symptoms can be suggestive of POTS when the patient also suffers from excessive orthostatic tachycardia, and that further, in-depth studies are needed to elucidate the duration of post-COVID CVAD, its underlying pathophysiology, and optimum treatment.
There is increasing evidence of incident cases of postural tachycardia following COVID-19. In an international online survey on 802 COVID-19 survivors who received a post-acute diagnosis, 19% reported receiving a POTS diagnosis [
54]. In this cohort, POTS was the second most common reported diagnosis in the aftermath of COVID-19, following migraine (27%).
4. How Does SARS-CoV-2 Infection Cause Long-Term Dysautonomia?
Infections are known triggers of dysautonomia, particularly in POTS. Among various mechanisms by which SARS-CoV-2 may induce dysautonomia, three possible conditions have been proposed and gained support from preliminary evidence: hypovolemia, brainstem involvement, and autoimmunity [
64].
Hypovolemia has been widely recognized in PASC patients with dysautonomic features [
25,
52]. Hypovolemia may trigger hyperadrenergic POTS and lead to cerebral hypoperfusion and the impairment of central autonomic networks [
56]. Preliminary evidence on post-COVID POTS supports the correction of hypovolemia by the liberal intake of water and salt [
16] and other nonpharmacological interventions aimed at intravascular volume expansion and increasing venous return [
65] (
Figure 1).
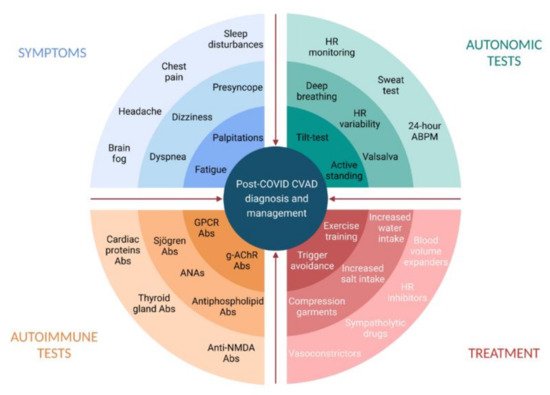
Figure 1. Post-COVID cardiovascular autonomic dysfunction: diagnosis and management. Abs, antibodies; ANAs, antinuclear antibodies; CVAD, cardiovascular autonomic dysfunction; g-AChR, ganglionic neuronal nicotinic acetylcholine receptor; GPCR, G-Protein coupled receptor; HR, heart rate. Created with BioRender.com.
Brainstem dysfunction can be associated with any clinical manifestation of PASC [
66], including autonomic failure [
67]. Proposed mechanisms for brainstem dysfunction following SARS-CoV-2 infection include direct viral invasion, neuroinflammation, vascular activation [
66], and brainstem compression [
56]. The nucleus tractus solitarii and reticular activation system have been involved in pathogenic hypotheses. Notably, a mild grade of brainstem dysfunction has been consistently demonstrated via magnetic resonance imaging in some conditions overlapping and comorbid to POTS, such as chronic fatigue syndrome [
68]. Brainstem dysfunction following COVID-19 has been found to be similar to that observed in takotsubo syndrome (TTS) [
64], and interestingly, a case for COVID-19-related TTS has recently been described [
69]. Although excessive endogenous stress hormone responses in the general population could likely explain the increased incidence of TTS during the pandemic [
70,
71], lesions of the brainstem, which has autonomic centers, have also been hypothesized as neural substrates [
72].
There is evidence suggesting a major role for autoimmunity in the pathophysiology of post-viral POTS [
73]. Patients with POTS have been shown to have a higher prevalence of specific autoantibodies (AAbs), including G-Protein coupled receptor (GPCR) antibodies, which could explain an increase in sympathetic tone by activating adrenergic receptors and eliciting a negative allosteric effect on muscarinic GPCRs [
74,
75]. These serum patterns have been confirmed in COVID-19 survivors with dysautonomia [
57] and are comparable to those seen in patients with CFS and small-fiber neuropathy [
56].
The autoimmune hypothesis can also explain the higher prevalence of symptoms in women, as prolonged estrogen exposure and infections are known to synergistically trigger autoimmunity [
76,
77].
Another possible explanation of coronavirus-induced CVAD is the direct damage of extracardiac postganglionic sympathetic neurons by the virus [
64]. However, there is currently no convincing evidence to support this hypothesis.
Finally, the role of excessive mast cell activation in the pathophysiology of POTS has been proposed. In mast cell activation disorder, the inappropriate release of histamine and other mast cell mediators in response to physical activity or orthostatic stress may lead to orthostatic tachycardia [
49]. Flushing, headaches, and gastrointestinal symptoms are more prevalent in these individuals, and a recent study showed markedly elevated prostaglandins and histamine markers in patients with gastrointestinal complaints, reinforcing the case for a mast cell activation disorder in a subset of POTS patients [
78].
5. Diagnosis of Post-COVID Dysautonomia
The diagnosis of post-COVID dysautonomia requires the comprehensive assessment of previous medical history, the careful evaluation and characterization of symptoms, and expert interpretation of neurological and cardiovascular autonomic investigations [
4,
16,
83].
Patients’ medical histories and their recollection of symptoms are essential in detecting the presence of dysautonomia [
84]. The histories of SARS-CoV-2 survivors with persistent autonomic dysfunction may reveal frequent fainting episodes, dizziness, lightheadedness, and/or palpitations [
63] prior to SARS-CoV-2 infection, revealing underlying hypotensive susceptibility or previous orthostatic intolerance syndromes. Frequent comorbidities include migraine, irritable bowel syndrome, chronic fatigue syndrome, and autoimmune diseases [
73,
77]. Lightheadedness, tachycardia, dyspnea, headache, and poor concentration are the most frequent symptoms in PASC patients. Fatigue is the single most frequent symptom, although it is not specific for dysautonomia [
33]. Severe symptoms may last for weeks or even months after infection and negatively affect overall quality of life [
23].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd8110156