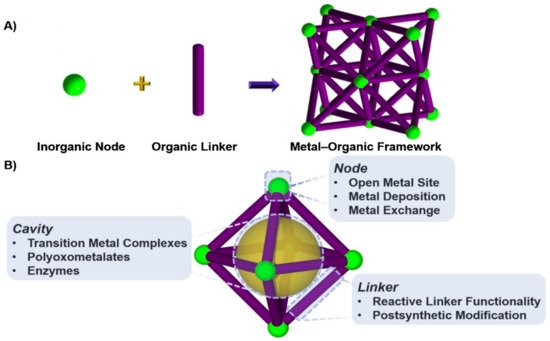
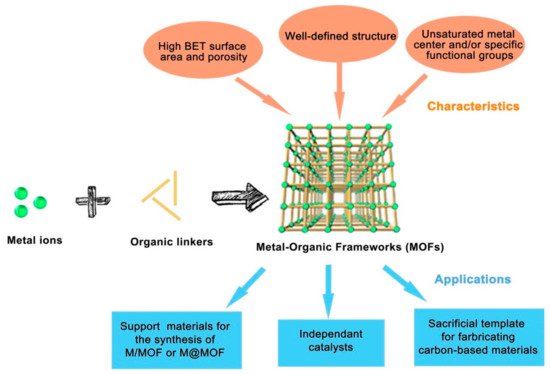
The level of carbon dioxide in the atmosphere is growing rapidly due to fossil fuel combustion processes, heavy oil, coal, oil shelter, and exhausts from automobiles for energy generation, which lead to depletion of the ozone layer and consequently result in global warming. The realization of a carbon-neutral environment is the main focus of science and academic researchers of today. Several processes were employed to minimize carbon dioxide in the air, some of which include the utilization of non-fossil sources of energy like solar, nuclear, and biomass-based fuels. Consequently, these sources were reported to have a relatively high cost of production and maintenance. The applications of both homogeneous and heterogeneous processes in carbon capture and storage were investigated in recent years and the focus now is on the conversion of CO2 into useful chemicals and compounds. It was established that CO2 can undergo cycloaddition reaction with epoxides under the influence of special catalysts to give cyclic carbonates, which can be used as value-added chemicals at a different level of pharmaceutical and industrial applications. Among the various catalysts studied for this reaction, metal-organic frameworks are now on the frontline as a potential catalyst due to their special features and easy synthesis. Several metal-organic framework (MOF)-based catalysts were studied for their application in transforming CO2 to organic carbonates using epoxides. Here, we report some recent studies of porous MOF materials and an in-depth discussion of two repeatedly used metal-organic frameworks as a catalyst in the conversion of CO2 to organic carbonates
- cycloaddition
- epoxides
- carbon dioxide
- metal-organic frameworks
1. Reaction Mechanism for the Production of Cyclic Carbonates from CO2 and Epoxides
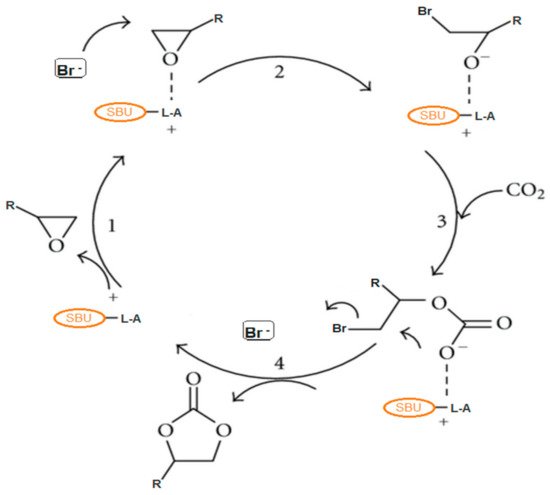
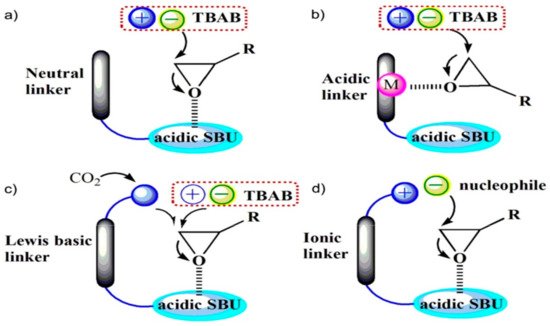
2. Metal-Organic Frameworks in CO2 Cycloaddition with Epoxides
| Entry | MOF Material | Co-Catalyst | Catalyst: Cocatalyst Loading (mol%) |
SBET (m2/g) |
Epoxide | Press (atm) |
Temp. (°C) | Time (h) | Selectivity (%) | Yield (%) |
Isosteric Heat Qst (Kj/Mol) |
Reusability | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Al(OH) (O2C–CH=CH–CO2)∙nH2O | TBABr | 0.02:0.002 | 1169 | ECH | 10 | 50 | 6 | 97 | 95 | 23 | 4 cycles | [60] |
| 2 | Zn2(Py)(Atz)2∙DMF∙2H2O | TBABr | 0.1:0.1 | 764.5 | PO | 15 | 60 | 4 | 98 | 92 | 27.7 | 6 cycles | [40] |
| 3 | [In2(L)(OH)2]·2DMF·2H2O | TBABr | 0.5:0.2 | 1022 | EBH | 1 | 70 | 12 | 89 | 99 | - | 5 cycles | [61] |
| 4 | F-Mn-MOF-74 | TBABr | 0.1:0.031 | 20.83 | SO | 10 | 100 | 6 | 99 | 99 | - | 7 cycles | [62] |
| 5 | PCN-222(Co)@MTTB | TBABr | 0.1:0.216 | PO/ECH | 1 | 50 | 20 | 98 | >98 | - | 3 cycles | [51] | |
| 6 | rho-ZMOF | TBABr | 0.1:1.4 | 871 | ECH | 10 | 40 | 3 | 98 | 98 | - | 5 cycles | [54] |
| 7 | Co-MOF-2 {[Co(BDC)(L)]·2H2O.xG}n |
TBABr | 1.8:2.5 | 6.8 | SO/ECH | 1 | 40 | 12 | 99 | 99 | 35.0 | 6 cycles | [44] |
| 8 | {[Zn(H2O)(HL)]⋅(DMF)2 (H2O)2}n | TBABr | 0.25:0.232 | 945 | PO | 1 | RT | 48 | - | 76 | - | - | [48] |
| 9 | MOF-5-MIX | TBABr | 0.5:0.5 | 357 | ECH | 12 | 50 | 6 | 99 | 98 | - | 5 cycles | [63] |
| 10 | Ce-NU-1008 | TBABr | 0.02:0.002 | SO | 1 | RT | 20 | 95 | - | 3 cycles | [64] | ||
| 11 | Co-MOF-2 {[Co(BDC)(L)]·2H2O·xG}n |
KI | 5.0:0.2 | 6.8 | SEO | 1 | 40 | 8 | 99 | 99 | 35.0 | 6 cycles | [65] |
| 12 | {[Ni3HL(μ3-OH)(H2O)2]∙3(H2O)∙DMA}n | TBABr | 0.025:1.5 | 743.5 | ECH | 10 | 100 | 6 | - | >99 | - | 5 cycles | [66] |
| 13 | [(Cu2 BPDSDC∙4DMF)∙2DMF]n | TBABr | 0.05:0.1 | - | PO | 25 | 80 | 5 | 98 | 99 | - | 4 cycles | [67] |
| 14 | {[Co6(OH)2(H2O)4 (cpt)9](NO3)(DMF)13} | TBABr | 0.1:2 | 873 | PO | 1 | 40 | 48 | 97 | 97 | 32 | 4 cycles | [68] |
| 15 | InDCPN-Cl | TBABr | 0.05:5.00 | 997 | SO | 1 | 80 | 24 | 98 | 93 | 30 | 5 cycles | [16] |
| 16 | Ce-NU-1008 | TBABr | 0.002:0.02 | 910 | SO | 1 | RT | 20 | 95 | - | - | [64] | |
| 17 | MOF-5@Imidazolium iodide | - | - | 277.9 | SO | 10 | 110 | 8 | - | 92 | - | 4 cycles | [69] |
| 18 | [(CH3)2NH2][M(COOH)3] | - | 13.1 | 13.11 | PO | 20 | 120 | 6 | 100 | 98 | - | 3 cycles | [70] |
| 19 | Im-MnF [C3H5N2][Mn(COOH)3] |
- | - | 81.57 | ECH | 15 | 100 | 6 | 99 | 95 | - | - | [71] |
| 20 | Pt/Mg-MOF-74 | - | 513 | PO | 17.5 | 150 | 4 | 77 | 44 | - | 3 cycles | [72] |
3. MIL-101 Based MOFs in CO2 Cycloaddition with Epoxides
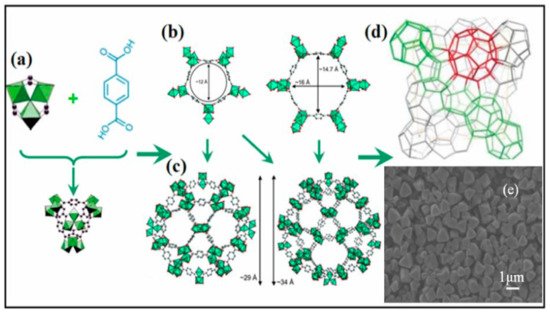
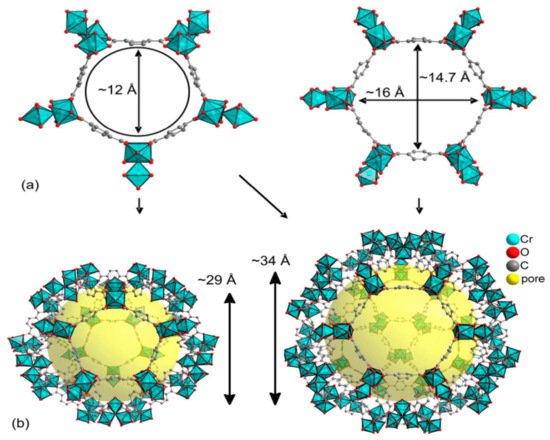
4. HKUST-1 for CO2 Cycloaddition with Epoxides
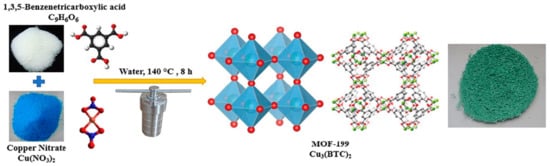
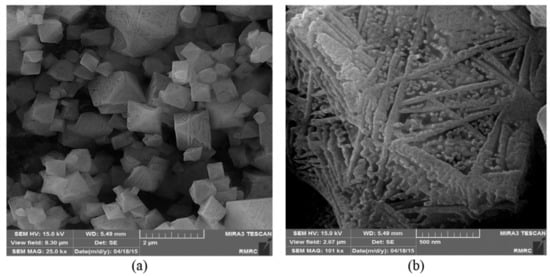
Abbreviations
| MOFs | Metal-organic frameworks |
| TBABr | Tetrabutyl ammonium bromide |
| SBU | Secondary Building Unit |
| SO | Styrene Oxide |
| PO | Propylene oxide |
| ECH | Epichlorohydrin |
| EBH | Epibromohydrin |
| SEO | Spiro-Epoxy Oxindole |
| BTC | 1,3,5-benzenetricarboxylate |
| HKUST | Hong Kong University of Science and Technology |
| RT | Room temperature |
| KI | potassium iodide |
| Å | Aperture |
This entry is adapted from the peer-reviewed paper 10.3390/polym13223905
References
- Beyzavi, M.H.; Stephenson, C.J.; Eliu, Y.; Ekaragiaridi, O.; Hupp, J.T.; Farha, O.K. Metal-Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2015, 3, 1–10.
- Pentyala, V.; Davydovskaya, P.; Pohle, R.; Urban, G.; Yurchenko, O. Mg-MOF74 and Co-MOF74 as Sensing Layers for CO2 Detection. Procedia Eng. 2014, 87, 1071–1074.
- Wu, Y.; Song, X.; Li, S.; Zhang, J.; Yang, X.; Shen, P.; Gao, L.; Wei, R.; Zhang, J.; Xiao, G. 3D-monoclinic M–BTC MOF (M = Mn, Co, Ni) as highly efficient catalysts for chemical fixation of CO2 into cyclic carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303.
- Lu, B.-B.; Jiang, W.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Resorcin arene-Based Microporous Metal–Organic Framework as an Efficient Catalyst for CO2 Cycloaddition with Epoxides and Highly Selective Luminescent Sensing of Cr2O72−. ACS Appl. Mater. Interfaces 2017, 9, 39441–39449.
- Lin, K.-S.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871.
- Tufa, R.A.; Chanda, D.; Ma, M.; Aili, D.; Demissie, T.B.; Vaes, J.; Li, Q.; Liu, S.; Pant, D. Towards highly efficient electrochemical CO2 reduction: Cell designs, membranes and electrocatalysts. Appl. Energy 2020, 277, 115557.
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal Organic Framework Based Catalysts for CO2 Conversion. Mater. Horizons 2017, 4, 345–361.
- Connolly, B.M.; Mehta, J.P.; Moghadam, P.Z.; Wheatley, A.E.; Fairen-Jimenez, D. From synthesis to applications: Metal–organic frameworks for an environmentally sustainable future. Curr. Opin. Green Sustain. Chem. 2018, 12, 47–56.
- Yu, B.; Zou, B.; Hu, C.-W. Recent applications of polyoxometalates in CO2 capture and transformation. J. CO2 Util. 2018, 26, 314–322.
- Chen, F.; Dong, T.; Chi, Y.; Xu, Y.; Hu, C. Transition-Metal-Substituted Keggin-Type Germanotungstates for Catalytic Conversion of Carbon Dioxide to Cyclic Carbonate. Catal. Lett. 2010, 139, 38–41.
- Huh, S. Direct Catalytic Conversion of CO2 to Cyclic Organic Carbonates under Mild Reaction Conditions by Metal—Organic Frameworks. Catalysts 2019, 9, 34.
- Beyzavi, M.H.; Klet, R.C.; Tussupbayev, S.; Borycz, J.; Vermeulen, N.A.; Cramer, C.J.; Stoddart, J.F.; Hupp, J.T.; Farha, O.K. A Hafnium-Based Metal–Organic Framework as an Efficient and Multifunctional Catalyst for Facile CO2 Fixation and Regioselective and Enantioretentive Epoxide Activation. J. Am. Chem. Soc. 2014, 136, 15861–15864.
- D’Elia, V.; Dong, H.; Rossini, A.J.; Widdifield, C.M.; Vummaleti, S.V.C.; Minenkov, Y.; Poater, A.; Abou-Hamad, E.; Pelletier, J.D.A.; Cavallo, L.; et al. Cooperative Effect of Monopodal Silica-Supported Niobium Complex Pairs Enhancing Catalytic Cyclic Carbonate Production. J. Am. Chem. Soc. 2015, 137, 7728–7739.
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal-Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003.
- Ugale, B.; Dhankhar, S.S.; Nagaraja, C.M. Exceptionally Stable and 20-Connected Lanthanide Metal–Organic Frameworks for Selective CO2 Capture and Conversion at Atmospheric Pressure. Cryst. Growth Des. 2018, 18, 2432–2440.
- Yuan, Y.; Li, J.; Sun, X.; Li, G.; Liu, Y.; Verma, G.; Ma, S. Indium-Organic Frameworks Based on Dual Secondary Building Units Featuring Halogen-Decorated Channels for Highly Effective CO2 Fixation. Chem. Mater. 2019, 31, 1084–1091.
- Nguyen, P.T.K.; Nguyen, H.N.; Trickett, C.A.; Ton, Q.T.; Gutiérrez-Puebla, E.; Monge, M.A.; Cordova, K.E.; Gándara, F. New Metal–Organic Frameworks for Chemical Fixation of CO2. ACS Appl. Mater. Interfaces 2017, 10, 733–744.
- Zhou, X.; Zhang, Y.; Yang, X.; Zhao, L.; Wang, G. Functionalized IRMOF-3 as efficient heterogeneous catalyst for the synthesis of cyclic carbonates. J. Mol. Catal. A Chem. 2012, 361–362, 12–16.
- Kiatkittipong, K.; Shukri, M.A.A.M.; Kiatkittipong, W.; Lim, J.W.; Show, P.L.; Lam, M.K.; Assabumrungrat, S. Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes 2020, 8, 548.
- Calabrese, C.; Giacalone, F.; Aprile, C. Hybrid Catalysts for CO2 Conversion into Cyclic Carbonates. Catalysts 2019, 9, 325.
- Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M.; et al. Step-by-Step Growth of HKUST-1 on Functionalized TiO2 Surface: An Efficient Material for CO2 Capture and Solar Photoreduction. Catalysts 2018, 8, 353.
- Liang, J.; Huang, Y.-B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65.
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450.
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315.
- Chen, Y.; Huang, X.; Feng, X.; Li, J.; Huang, Y.; Zhao, J.; Guo, Y.; Dong, X.; Han, R.; Qi, P.; et al. Facile fabrication of magnetically recyclable metal–organic framework nanocomposites for highly efficient and selective catalytic oxidation of benzylic C–H bonds. Chem. Commun. 2014, 50, 8374–8377.
- Yang, Y.; Yao, H.-F.; Xi, F.-G.; Gao, E.-Q. Amino-functionalized Zr(IV) metal–organic framework as bifunctional acid–base catalyst for Knoevenagel condensation. J. Mol. Catal. A Chem. 2014, 390, 198–205.
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425.
- Wasson, M.C.; Buru, C.T.; Chen, Z.; Islamoglu, T.; Farha, O.K. Metal–organic frameworks A tunable platform to access single-site heterogeneous catalysts. Appl. Catal. A Gen. 2019, 586, 117214.
- Du, P.D.; Thanh, H.T.M.; To, T.C.; Thang, H.S.; Tinh, M.X.; Tuyen, T.N.; Hoa, T.T.; Khieu, D.Q. Metal-Organic Framework MIL-101: Synthesis and Photocatalytic Degradation of Remazol Black B Dye. J. Nanomater. 2019, 2019, 6061275.
- Uba, Z.Z.; Hana, N.; Abu, H.; Soraya, N.; Jumbri, K.; Ain, N.; Abdullah, F.; Abdul, E.; Saad, B. Adsorption of Chrysene in Aqueous Solution onto MIL-88(Fe) and NH2-MIL-88(Fe) Metal-Organic Frameworks: Kinetics, Isotherms, Thermodynamics and Docking Simulation Studies. J. Environ. Chem. Eng. 2019, 88, 103544.
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280.
- Zhao, X.; Wei, Y.; Zhao, H.; Gao, Z.; Zhang, Y.; Zhi, L.; Wang, Y.; Huang, H. Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions. J. Colloid Interface Sci. 2018, 514, 234–239.
- Seo, P.W.; Khan, N.A.; Jhung, S.H. Removal of nitroimidazole antibiotics from water by adsorption over metal–organic frameworks modified with urea or melamine. Chem. Eng. J. 2017, 315, 92–100.
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413.
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Abu Bakar, N.H.H.; Abdullah, N.A.F.; Negim, E.-S.M.; Saad, B. Experimental and molecular docking model studies for the adsorption of polycyclic aromatic hydrocarbons onto UiO-66(Zr) and NH2-UiO-66(Zr) metal-organic frameworks. Chem. Eng. Sci. 2020, 220, 115608.
- Zhang, X.; Zhang, N.; Gan, C.; Liu, Y.; Chen, L.; Zhang, C.; Fang, Y. Synthesis of In2S3/UiO-66 hybrid with enhanced photocatalytic activity towards methyl orange and tetracycline hydrochloride degradation under visible-light irradiation. Mater. Sci. Semicond. Process. 2019, 91, 212–221.
- Wu, T.; Yan, T.; Zhang, X.; Feng, Y.; Wei, D.; Sun, M.; Du, B.; Wei, Q. A competitive photoelectrochemical immunosensor for the detection of diethylstilbestrol based on an Au/UiO-66(NH2)/CdS matrix and a direct Z-scheme Melem/CdTe heterojunction as labels. Biosens. Bioelectron. 2018, 117, 575–582.
- Niknam, E.; Panahi, F.; Daneshgar, F.; Bahrami, F.; Khalafi-Nezhad, A. Metal–Organic Framework MIL-101(Cr) as an Efficient Heterogeneous Catalyst for Clean Synthesis of Benzoazoles. ACS Omega 2018, 3, 17135–17144.
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deep, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66.
- Lan, J.; Qu, Y.; Zhang, X.; Ma, H.; Xu, P.; Sun, J. A novel water-stable MOF Zn(Py)(Atz) as heterogeneous catalyst for chemical conversion of CO2 with various epoxides under mild conditions. J. CO2 Util. 2020, 35, 216–224.
- Pawar, A.A.; Kim, H. Reaction parameters dependence of the CO2/epoxide coupling reaction catalyzed by tunable ionic liquids, optimization of comonomer-alternating enhancement pathway. J. CO2 Util. 2019, 33, 500–512.
- Hu, L.; Yan, Z.; Mo, X.; Peng, X.; Chen, L. Hierarchical Co/ZIF-8 as an efficient catalyst for cycloaddition of CO2 and epoxide. Microporous Mesoporous Mater. 2020, 294, 109917.
- Lan, J.; Qu, Y.; Xu, P.; Sun, J. Novel HBD-Containing Zn (dobdc) (datz) as efficiently heterogeneous catalyst for CO2 chemical conversion under mild conditions. Green Energy Environ. 2020, 6, 66–74.
- Patel, P.; Parmar, B.; Pillai, R.S.; Ansari, A.; Khan, N.-U.H.; Suresh, E. CO2 fixation by cycloaddition of mono/disubstituted epoxides using acyl amide decorated Co(II) MOF as a synergistic heterogeneous catalyst. Appl. Catal. A Gen. 2020, 590, 117375.
- Gupta, A.K.; Guha, N.; Krishnan, S.; Mathur, P.; Rai, D.K. A Three-Dimensional Cu(II)-MOF with Lewis acid-base dual functional sites for Chemical Fixation of CO2 via Cyclic Carbonate Synthesis. J. CO2 Util. 2020, 39, 101173.
- Gallardo-Fuentes, S.; Contreras, R.; Isaacs, M.; Honores, J.; Quezada, D.; Landaeta, E.; Ormazábal-Toledo, R. On the mechanism of CO2 electro-cycloaddition to propylene oxides. J. CO2 Util. 2016, 16, 114–120.
- Wu, Y.; Song, X.; Zhang, J.; Xu, S.; Xu, N.; Yang, H.; Miao, Y.; Gao, L.; Zhang, J.; Xiao, G. Zn2(C9H3O6)(C4H5N2)(C4H6N2)3 MOF as a highly efficient catalyst for chemical fixation of CO2 into cyclic carbonates and kinetic studies. Chem. Eng. Res. Des. 2018, 140, 273–282.
- Cai, Z.; Qian, J.; Zhao, Y.; Cheng, Y. Microporous MOF with open metal sites for CO2 fixation and protective effect on osteoarthritis by regulating the activation of PI3K/AKT pathway. J. Solid State Chem. 2020, 283, 121169.
- Zanon, A.; Chaemchuen, S.; Mousavi, B.; Verpoort, F. 1 Zn-doped ZIF-67 as catalyst for the CO2 fixation into cyclic carbonates. J. CO2 Util. 2017, 20, 282–291.
- Ciprian, M.; Ruiz, K.H.; Kassymova, M.; Wang, T.; Zhuiykov, S.; Chaemchuen, S.; Tu, R.; Verpoort, F. 3D derived N-doped carbon matrix from 2D ZIF-L as an enhanced stable catalyst for chemical fixation. Microporous Mesoporous Mater. 2019, 285, 80–88.
- Liu, E.; Zhu, J.; Yang, W.; Liu, F.; Huang, C.; Yin, S. PCN-222(Co) Metal–Organic Framework Nanorods Coated with 2D Metal–Organic Layers for the Catalytic Fixation of CO2 to Cyclic Carbonates. ACS Appl. Nano Mater. 2020, 3, 3578–3584.
- Dhankhar, S.S.; Ugale, B.; Nagaraja, C.M. Co-Catalyst-Free Chemical Fixation of CO2 into Cyclic Carbonates by using Metal-Organic Frameworks as Efficient Heterogeneous Catalysts. Chem. Asian J. 2020, 15, 2403–2427.
- Norouzi, F.; Khavasi, H.R. Diversity-Oriented Metal Decoration on UiO-Type Metal–Organic Frameworks: An Efficient Approach to Increase CO2 Uptake and Catalytic Conversion to Cyclic Carbonates. ACS Omega 2019, 4, 19037–19045.
- Zhang, S.; Jang, M.-S.; Lee, J.; Puthiaraj, P.; Ahn, W.-S. Zeolite-Like Metal Organic Framework (ZMOF) with a rho Topology for a CO2 Cycloaddition to Epoxides. ACS Sustain. Chem. Eng. 2020, 8, 7078–7086.
- Dhankhar, S.S.S.; Nagaraja, C.M. Construction of 3D lanthanide based MOFs with pores decorated with basic imidazole groups for selective capture and chemical fixation of CO2. New J. Chem. 2020, 44, 9090–9096.
- Hwang, G.-Y.; Roshan, R.; Ryu, H.-S.; Jeong, H.-M.; Ravi, S.; Kim, M.-I.; Park, D.-W. A highly efficient zeolitic imidazolate framework catalyst for the co-catalyst and solvent free synthesis of cyclic carbonates from CO2. J. CO2 Util. 2016, 15, 123–130.
- Liu, X.; Tang, B.; Long, J.; Zhang, W.; Liu, X.; Mirza, Z. The development of MOFs-based nanomaterials in heterogeneous organocatalysis. Sci. Bull. 2018, 63, 502–524.
- Hu, T.-D.; Jiang, Y.; Ding, Y.-H. Computational screening of metal-substituted HKUST-1 catalysts for chemical fixation of carbon dioxide into epoxides. J. Mater. Chem. A 2019, 7, 14825–14834.
- Kim, J.; Kim, S.-N.; Jang, H.-G.; Seo, G.; Ahn, W.-S. CO2 cycloaddition of styrene oxide over MOF catalysts. Appl. Catal. A Gen. 2013, 453, 175–180.
- Kim, H.S.; Yu, K.; Puthiaraj, P.; Ahn, W.-S. CO2 cycloaddition to epichlorohydrin over an aluminum fumarate metal-organic framework synthesized by a sonochemical route. Microporous Mesoporous Mater. 2020, 306, 110432.
- Zhang, B.; Guo, P.-Y.; Ma, L.-N.; Liu, B.; Hou, L.; Wang, Y.-Y. Two Robust In(III)-Based Metal–Organic Frameworks with Higher Gas Separation, Efficient Carbon Dioxide Conversion, and Rapid Detection of Antibiotics. Inorg. Chem. 2020, 59, 5231–5239.
- Feng, C.; Qiao, S.; Guo, Y.; Xie, Y.; Zhang, L.; Akram, N.; Li, S.; Wang, J. Adenine-assisted synthesis of functionalized F-Mn-MOF-74 as an efficient catalyst with enhanced catalytic activity for the cycloaddition of carbon dioxide. Colloids Surf. A Physicochem. Eng. ASP 2020, 597, 124781.
- Kurisingal, J.F.; Rachuri, Y.; Gu, Y.; Choe, Y.; Park, D.-W. Multi-variate metal organic framework as efficient catalyst for the cycloaddition of CO2 and epoxides in a gas-liquid-solid reactor. Chem. Eng. J. 2020, 386, 121700.
- Lyu, J.; Zhang, X.; Li, P.; Wang, X.; Buru, C.T.; Bai, P.; Guo, X.; Farha, O.K. Exploring the Role of Hexanuclear Clusters as Lewis Acidic Sites in Isostructural Metal–Organic Frameworks. Chem. Mater. 2019, 31, 4166–4172.
- Parmar, B.; Patel, P.; Pillai, R.S.; Tak, R.K.; Kureshy, R.I.; Khan, N.-U.H.; Suresh, E. Cycloaddition of CO2 with an Epoxide-Bearing Oxindole Scaffold by a Metal–Organic Framework-Based Heterogeneous Catalyst under Ambient Conditions. Inorg. Chem. 2019, 58, 10084–10096.
- Yang, Y.; Guo, Z.; Chen, X.-H.; Liu, J. A Ni3O-cluster based porous MOF for catalytic conversion of CO2 to cyclic carbonates. J. Solid State Chem. 2019, 276, 190–193.
- Guo, F. A novel 2D Cu(II)-MOF as a heterogeneous catalyst for the cycloaddition reaction of epoxides and CO2 into cyclic carbonates. J. Mol. Struct. 2019, 1184, 557–561.
- Chen, D.-M.; Zhang, X.-J. A polyhedron-based metal-organic framework with a rare hexanuclear Co(II) cluster for selective sorption and chemical conversion for CO2. J. Solid State Chem. 2019, 278, 120906.
- Mohammadian, R.; Kamyar, N.; Kaffashian, A.; Amini, M.M.; Shaabani, A. Synthesis of Defect-Engineered Homochiral Metal-Organic Frameworks Using L-Amino Acids: A Comprehensive Study of Chiral Catalyst Performance in CO2 Fixation Reaction. ChemistrySelect 2020, 5, 10346–10354.
- Wu, Y.; Song, X.; Xu, S.; Yu, T.; Zhang, J.; Qi, Q.; Gao, L.; Zhang, J.; Xiao, G. (M=Mn, Co, Ni, Zn) MOFs as highly efficient catalysts for chemical fixation of CO2 and DFT studies. Mol. Catal. 2019, 475, 110485.
- Wu, Y.; Song, X.; Zhang, J.; Xu, S.; Gao, L.; Zhang, J.; Xiao, G. Mn-based MOFs as efficient catalysts for catalytic conversion of carbon dioxide into cyclic carbonates and DFT studies. Chem. Eng. Sci. 2019, 201, 288–297.
- Abdelsayed, V.; Gardner, T.H.; Kababji, A.H.; Fan, Y. Catalytic conversion of CO2 to propylene carbonate over Pt-decorated Mg-substituted metal organic framework. Appl. Catal. A Gen. 2019, 586, 117225.
- Sankar, M.; Tarte, N.; Manikandan, P. Effective catalytic system of zinc-substituted polyoxometalate for cycloaddition of CO2 to epoxides. Appl. Catal. A Gen. 2004, 276, 217–222.
- Alivand, M.S.; Shafiei-Alavijeh, M.; Tehrani, N.H.M.H.; Ghasemy, E.; Rashidi, A.; Fakhraie, S. Facile and high-yield synthesis of improved MIL-101(Cr) metal-organic framework with exceptional CO2 and H2S uptake; the impact of excess ligand-cluster. Microporous Mesoporous Mater. 2019, 279, 153–164.
- Huang, X.; Hu, Q.; Gao, L.; Hao, Q.; Wang, P.; Qin, D. Adsorption characteristics of metal–organic framework MIL-101(Cr) towards sulfamethoxazole and its persulfate oxidation regeneration. RSC Adv. 2018, 8, 27623–27630.
- Yin, D.; Li, C.; Ren, H.; Shekhah, O.; Liu, J.; Liang, C. Efficient (Cr) hetero-catalysts for 2-butyne-1,4-diol hydrogenation exhibiting high selectivity. RSC Adv. 2017, 7, 1626–1633.
- Leng, K.; Sun, Y.; Li, X.; Sun, S.; Xu, W. Rapid Synthesis of Metal–Organic Frameworks MIL-101(Cr) Without the Addition of Solvent and Hydrofluoric Acid. Cryst. Growth Des. 2016, 16, 1168–1171.
- Chen, M.; Zhou, S.; Xu, Z.; Ding, L.; Cheng, Y. Metal-organic frameworks of MIL-100(Fe, Cr) and MIL-101(Cr) for Aromatic Amines Adsorption from Aqueous Solutions. Molecules 2019, 24, 3718.
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174.
- Zhao, T.; Jeremias, F.; Boldog, I.; Nguyen, B.; Henninger, S.K.; Janiak, C. High-yield, fluoride-free and large-scale synthesis of MIL-101(Cr). Dalton Trans. 2015, 44, 16791–16801.
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209–210, 151–157.
- Hupp, J.T.; Poeppelmeier, K.R. CHEMISTRY: Enhanced: Better Living through Nanopore Chemistry. Science 2005, 309, 2008–2009.
- Ertas, I.E.; Gulcan, M.; Bulut, A.; Yurderi, M.; Zahmakiran, M. Metal-organic framework (MIL-101) stabilized ruthenium nanoparticles: Highly efficient catalytic material in the phenol hydrogenation. Microporous Mesoporous Mater. 2016, 226, 94–103.
- Zhao, Z.; Wang, S.; Liang, R.; Li, Z.; Shi, Z.; Chen, G. Graphene-wrapped chromium-MOF(MIL-101)/sulfur composite for performance improvement of high-rate rechargeable Li–S batteries. J. Mater. Chem. A 2014, 2, 13509–13512.
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81.
- Gordon, J.; Kazemian, H.; Rohani, S. MIL-53(Fe), MIL-101, and SBA-15 porous materials: Potential platforms for drug delivery. Mater. Sci. Eng. C 2015, 47, 172–179.
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374.
- Juan-Alcañiz, J.; Ramos-Fernandez, E.V.; Lafont, U.; Gascon, J.; Kapteijn, F. Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts. J. Catal. 2010, 269, 229–241.
- Hu, X.; Lu, Y.; Dai, F.; Liu, C.; Liu, Y. Host–guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via “bottle around ship”: An effective catalyst for oxidative desulfurization. Microporous Mesoporous Mater. 2013, 170, 36–44.
- Akimana, E.; Wang, J.; Likhanova, N.V.; Chaemchuen, S.; Verpoort, F. MIL-101(Cr) for CO2 Conversion into Cyclic Carbonates, Under Solvent and Co-Catalyst Free Mild Reaction Conditions. Catalysts 2020, 10, 453.
- Peng, L.; Zhang, J.; Xue, Z.; Han, B.; Sang, X.; Liu, C.; Yang, G. Highly mesoporous metal–organic framework assembled in a switchable solvent. Nat. Commun. 2014, 5, 4465.
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442.
- Murray, L.J.; Dinca, M.; Yano, J.; Chavan, S.; Bordiga, S.; Brown, C.M.; Long, J.R. Highly-Selective and Reversible O2 Binding in Cr3(1,3,5-benzenetricarboxylate)2. J. Am. Chem. Soc. 2010, 132, 7856–7857.
- Maniam, P.; Stock, N. Investigation of Porous Ni-Based Metal–Organic Frameworks Containing Paddle-Wheel Type Inorganic Building Units via High-Throughput Methods. Inorg. Chem. 2011, 50, 5085–5097.
- Feldblyum, J.I.; Liu, M.; Gidley, D.W.; Matzger, A.J. Reconciling the Discrepancies between Crystallographic Porosity and Guest Access as Exemplified by Zn-HKUST-1. J. Am. Chem. Soc. 2011, 133, 18257–18263.
- Zhang, Z.; Zhang, L.; Wojtas, L.; Eddaoudi, M.; Zaworotko, M.J. Template-Directed Synthesis of Nets Based upon Octahemioctahedral Cages That Encapsulate Catalytically Active Metalloporphyrins. J. Am. Chem. Soc. 2011, 134, 928–933.
- Crane, J.L.; Anderson, K.E.; Conway, S.G. Hydrothermal Synthesis and Characterization of a Metal–Organic Framework by Thermogravimetric Analysis, Powder X-ray Diffraction, and Infrared Spectroscopy: An Integrative Inorganic Chemistry Experiment. J. Chem. Educ. 2014, 92, 373–377.
- Gotthardt, M.A.; Schoch, R.; Wolf, S.; Bauer, M.; Kleist, W. Synthesis and characterization of bimetallic metal–organic framework Cu–Ru-BTC with HKUST-1 structure. Dalton Trans. 2014, 44, 2052–2056.
- Raganati, F.; Gargiulo, V.; Ammendola, P.; Alfe, M.; Chirone, R. CO2 capture performance of HKUST-1 in a sound assisted fluidized bed. Chem. Eng. J. 2014, 239, 75–86.
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. 2018, 28, 584–589.
- Functionalizable, A.C.; Material, N.; Tma, C.; Chui, S.S.; Lo, S.M.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D.; Ho, C.T.M.A.; Chui, S.S.; et al. Published by American Association for the Advancement of Science Linked References Are Available on JSTOR for This Article. Nanoporous Mater. 2016, 283, 1148–1150.
- O’Neill, L.D.; Zhang, H.; Bradshaw, D. Macro-/Microporous MOF Composite Beads. J. Mater. Chem. 2010, 20, 5720–5726.
- Xin, C.; Jiao, X.; Yin, Y.; Zhan, H.; Li, H.; Li, L.; Zhao, N.; Xiao, F.; Wei, W. Enhanced CO2 Adsorption Capacity and Hydrothermal Stability of HKUST-1 via Introduction of Siliceous Mesocellular Foams (MCFs). Ind. Eng. Chem. Res. 2016, 55, 7950–7957.
- MacIas, E.E.; Ratnasamy, P.; Carreon, M.A. Catalytic Activity of Metal Organic Framework Cu3(BTC)2 in the Cycloaddition of CO2 to Epichlorohydrin Reaction. Catal. Today 2012, 198, 215–218.
- Rani, D.; Kumar, R.; Kumar, V.; Singh, M. High yield cycloaddition of carbon dioxide to epoxides catalyzed by metal–organic frameworks. Mater. Today Sustain. 2019, 5, 100021.
