Pharmaceutical manufacturing is evolving from traditional batch processes to continuous ones. The present review deals with the most recent technologies, based on spray freeze-drying, that can achieve this objective. It provides a comprehensive overview of the physics behind this process and of the most recent equipment design.
- spray freeze-drying
- freezing
- atomization
- pharmaceuticals
- continuous manufacturing
Pharmaceutical manufacturing is evolving from traditional batch processes to continuous ones. The new global competition focused on throughput and quality of drug products is certainly the driving force behind this transition which, thus, represents the new challenge of pharmaceutical manufacturing and hence of lyophilization as a downstream operation.
- Introduction
The current worldwide problems with the coronavirus pandemic have demonstrated the fragility of the pharmaceutical supply chain. The implementation of continuous manufacturing of biopharmaceuticals would offer tremendous benefits, providing a more agile and reliable supply chain for both existing and new products, that can rapidly address emergencies. Despite having witnessed the development of continuous technologies for the manufacturing of specific products, many barriers remain to the formation of an overall strategy [1,2]. For example, the transition from the consolidated batch technology to a completely new strategy is hindered by the lack of personnel expertise and training. However, there is a great opportunity in the adoption of continuous platforms for the new generation of drug products, i.e., biopharmaceuticals [3].
Biopharmaceuticals are large and complex molecules that need to be treated with caution to maintain their effectiveness. The relatively short shelf-life stability of liquid biomolecular-based drugs, as well as cold chain storage and transport costs, makes it necessary to remove water by drying. Even though all drying techniques share a common goal, they are conceptually different and not always suitable to deal with temperature-sensitive products. Furthermore, although most of the drying techniques were designed for batch processes, some of them, such as spray freeze-drying (SFD), could theoretically be adapted to serve the continuous drug production.
SFD is a technique that takes advantage of characteristics of spray drying, which involves the atomization of a liquid to create smaller particles, and freeze-drying, which has particular value for drying thermally sensitive materials, to create dry powders in controlled size and enhanced stability. It has recently been proposed as a way for the production of powders with specific targets, preparing formulations of BCS Class II drugs to improve their dissolution behavior (e.g., phenytoin, ciclosporin, danazol, carbamazepine and THC), developing inhalable dry powders for drug substances that do not fall in the categories mentioned above (e.g., voriconazole), or for some necessary dosage forms (e.g., needle-free injection or pulmonary application) [4]. On the other hand, the disadvantages are that fast cooling promotes the formation of a large ice–glass interface at which protein denaturation can easily occur. At the same time, too low cooling rates might indeed result in phase separation. For this reason, an intermediate cooling rate of about 1 °C/min is recommended [5]. The use of excipients, for instance sugars that provide stabilization by means of vitrification and water replacement, is often necessary to prevent or minimize loss of biological activity during processing [6]. A limited number of organic solvents can be used in SFD, as a high vapor pressure and relatively high melting temperature (or eutectic temperature when mixtures with water are used) are needed to yield an acceptable processing time. On the other hand, from an industry point of view, this technology can effectively respond to the new challenge of pharmaceutical manufacturing; that is, moving from batch to continuous operations in order to meet the lack of effective manufacturing capacity and plants. From an industrial point of view, even if SFD is developing slowly, it might become part of the pharmaceutical industry standard. From this perspective, this work will give a comprehensive review of the current and potential technological applications of SFD, as well as a general discussion on how some recent advances in computational approaches and mathematical modelling may enhance the understanding of the processes taking place at scale of the porous matrix and of the influence of its complex structure [7,8]; the methodologies described in these recent works are now mature enough to construct reliable computational models for investigating those pore-scale dynamics which can be difficult to explore with experiments. These insights can be very useful for the equipment design, e.g., by giving insight into the freezing process and its impact on product morphology [9], or enhancing professionals in solving practical and technical issues or tackling complex problems, which are now slowing the development of continuous freeze-drying operations.
The freeze-drying process is constituted by different spatial scales and process steps. Thus, one obstacle to the reliable industrial exploitation of a fully computational approach is the difficulty in finding a link between models at different scales of resolution or simply referring to a different portion of the process, and in ensuring a coherent flow of information between them. This concept of connection between different temporal and spatial modelling scales is what is usually referred to as model interoperability, which is the necessary premise for the full exploitation of modelling workflows in the industry [10,11].
The purpose of the current review is, thus, to give a comprehensive discussion of both advantages and challenges of SFD as a continuous operation, presenting the new opportunities given by the most recent technologies.
- Principles of Spray Freeze-Drying
The concept of SFD dates back to the late 1940s, when Benson and Ellis [12] successfully applied the process to produce protein particles having different values of specific surface area. Their process concept included the spray freezing of solution into a liquid nitrogen bath, followed by vacuum freeze-drying. SFD only started to be actively investigated in the 1990s [13], although its application was first demonstrated in the late 1950s by Meryman, who showed for the first time the possibility of freeze-drying at atmospheric pressure [14].
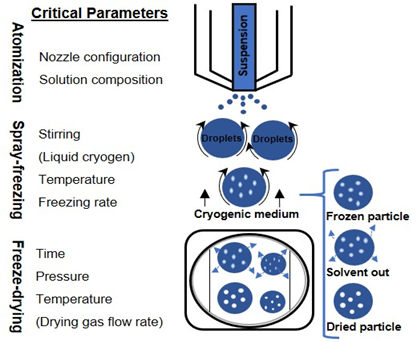
The SFD technique is a three-step operation [15]. The feed solution is initially sprayed through an atomizer, and the atomized droplets are frozen by contact with the low-temperature medium, locking in the spherical droplet shape. After cryogenic processing, the frozen droplets are transferred into a freeze-dryer to sublime water [16] and obtain a dried powder. While the physical and morphological properties of the obtained powder are mainly affected by the first two stages, the final stage relates to the drying time and the total energy required to dry the product [17]. This technique allows a high degree of control over the residual moisture content, mass density, and particle size; in fact, it allows easy manipulation of process parameters such as temperature of the cryogenic liquid, chemical composition and concentration of the solution, and the choice of the atomizer type [18].
The design and operation of each stage are discussed in the following sections, focusing on the atomization (type and size of the nozzle, and nominal flow rate), drying conditions, and formulations. SFD has been increasingly used as a process for pharmaceutical production: the current and proposed new applications of SFD are listed in Table 1.
Table 1. Examples of spray freeze-drying techniques reported in the literature for different purposes: the active ingredient and carrier for each application are listed.
|
Active |
Carrier |
Application/Purpose |
SFD Technique |
Main Findings |
Reference |
|
Voriconazole |
Mannitol |
Pulmonary delivery |
SFV/L + conventional FD |
SFD powder at an equivalent dose of voriconazole showed improved dissolution rate and lung deposition in comparison with liquid voriconazole. |
[19] |
|
Naked plasmid DNA (pDNA) |
Polyethylenimine (PEI), hyaluronic acid (LHA) |
Pulmonary gene therapy |
SFV/L + conventional FD |
The use of LHA showed the higher gene expression of pDNA in the murine lungs. The optimizing excipients resulted in good inhalation of porous 10 μm diameter naked pDNA powder. |
[20] |
|
Adalimumab |
Trehalose, amino acids (leucine, phenylalanine, glycine, or arginine) |
Pulmonary delivery |
SFV/L + conventional FD |
A combination of leucine or phenylalanine into adalimumab formulations increased the aerodynamic performance. Adding amino acids was found to increase for long-term stability. |
[21] |
|
SHetA2 |
Trehalose |
Oral administration |
SFV/L + conventional FD |
SHetA2-Kolliphor HS 15 complex was highly porous with a volume diameter of 23.54 ± 0.68 μm, and the apparent maximum solubility was enhanced in SHetA2 powders. |
[22] |
|
Naproxen |
Lactose |
Drug solubility |
SFV/L + conventional FD |
Although SFD nanoparticles were aggregated, their disaggregation was better than the spray dried ones of the same composition reported in the literature. The initial nanodispersion pH was also found to be highly effective in the re-dispersion. |
[23] |
|
Lysozyme |
Sucrose, mannitol |
Pulmonary delivery |
SFV/L + conventional FD |
Formulations prepared via spray freeze-drying and spray drying showed significant differences in the aerosol performance: spray dried small and dense particles compared to spray freeze-dried large and low-density particles. Multiple linear regression analysis was used to understand the relationship between powder properties, device dispersion mechanism, and aerosol performance. |
[24] |
|
Small interfering RNA (siRNA) |
Polyethyleneimine (PEI) |
Pulmonary gene therapy |
SFV/L + conventional FD |
SFD produced siRNA/PEI powder with high aerosol performance, and the potential for using the pulmonary delivery of the powder was demonstrated by specific and dose-dependent gene silencing activity against tumors in the lungs of mice. |
[25] |
|
Immunoglobulin G (IgG) |
Trehalose, amino acids (leucine, phenylalanine, arginine, cysteine, and glycine) |
Protein stability |
SFV/L + conventional FD |
The formulation results showed that the trehalose, combined with phenylalanine, gave better stabilization to IgG against shear, freezing, and dehydration stresses during SFD. |
[26] |
|
Raloxifene hydrochloride (RH) |
Polyvinylpyrrolidone, hydroxypropyl-β-cyclodextrin |
Drug solubility |
SFV/L + conventional FD |
The powder prepared using SFD exhibited good solubility and dissolution without affecting the chemical structure of RH. |
[27] |
|
Small interfering RNA (siRNA) |
Mannitol |
Pulmonary delivery |
SFV/L + conventional FD |
It was observed that the atomization gas flow rate had a significant effect on aerosol properties, whereas liquid feed rate had a small effect, and increased gas flow rate provided better aerosol performance due to a reduction in particle size. |
[28] |
|
Lysozyme |
Mannitol, sucrose, histidine |
Pulmonary delivery |
SFV/L + conventional FD |
Systematic rapid screening methods were developed for rapid formulations of powders to achieve desired aerosol performance and long-term stability. |
[6] |
|
Azithromycin |
Polyvinyl alcohol |
Drug solubility |
SFV/L + conventional FD |
The use of polymer resulted in increased solubility and dissolution of azithromycin more than pure drugs obtained by the SFD technique. |
[29] |
|
Clarithromycin |
Mannitol, sucrose |
Pulmonary delivery |
SFV/L + conventional FD |
SFD produced porous particles and improved the aerosolization efficiency of liposomal dry powders. |
[30] |
|
Lysozyme |
Bovine serum albumin, maltodextrin polyvinylpyrrolidone, mannitol |
Formulation development |
SFV/L + conventional FD |
Demonstrated that SFD allowed obtaining spherical, porous, and free-flowing particles for various therapeutic applications. |
[31] |
|
Budesonide |
Mannitol, cyclodextrin, leucine |
Inhalation therapy |
SFV/L + conventional FD |
Demonstrated that the formulation strategies using excipients resulted in a different aerosol performance of powder. |
[32] |
|
Hepatitis B vaccine |
Dextran, trehalose, inulin |
Drug stability |
SFV/L + conventional FD |
The presence of inulin or a combination of dextran and trehalose in an HBsAg formulation gave more stable product than the existing GlaxoSmithKline product containing aluminum hydroxide |
[33] |
|
Kanamycin |
- |
Pulmonary delivery |
SFL + conventional FD |
After optimizing the SFD procedure for the use of kanamycin drug, porous powders with good aerodynamic properties with a diameter of 13.5 μm were obtained. |
[34] |
|
Lactate dehydrogenase |
Trehalose |
Protein stability |
SFL, SFV/L + conventional FD |
Although similar powder surface areas were obtained in both cases, the SFL technique resulted in higher enzyme activities than SFD. |
[35] |
|
Bovine serum albumin |
Trehalose |
Powder processing |
SFV + conventional FD |
Free-flowing and porous powders with good aerodynamic properties were obtained by the combination of spray freezing step and fluidization conveying of powder using co-current flow. |
[36] |
|
Bovine serum albumin |
Trehalose |
Drug stability |
SFL + conventional FD |
A comparison of produced particles by SFV/L and SFL showed that denaturation and aggregation of protein were reduced in SFL relative to SFD. |
[37] |
|
Albuterol sulfate |
Polyethylene glycol |
Drug delivery |
SFL + conventional FD |
Spherical particle shape of median diameter ranging from 25 µm to 600 µm obtained by varying the processing parameters. |
[38] |
|
Carbamazepine |
Poloxamer 407, polyvinylpyrrolidone K15 |
Drug solubility |
SFL + conventional FD |
The use of the acetonitrile system in the SFL technique was found to be more effective in enhancing particle dissolution rate. |
[39] |
|
Danazol, hydroxypropyl-β-cyclodextrin |
Cyclodextrin |
Drug solubility |
SFL + conventional FD |
Flowable microencapsulated powders having high surface area and better dissolution profiles were obtained. |
[40] |
* SFV/L: Spray freezing into vapor over liquid, SFL: Spray freezing into liquid, FD: Freeze-drying.
- Spray Freezing Techniques
In SFD, the quench into a cryogen provides rapid freezing and thus mitigates the undesirable effects of large ice crystals, pH shift, and phase separation of drug and excipients. Among the different types of cryogens, e.g., liquid argon, propane, pentane, carbon dioxide, the ones most used are liquid and gas nitrogen. The reasons for this can be found in its relative inertness, density and viscosity, which vary considerably with pressure and temperature, and its desirable low boiling point (−196 °C) [35,38,71,72].
Freezing takes place very quickly as a consequence of the very low temperature of the cryogenic medium and the increased surface area of the droplets formed during the spraying phase. Thanks to their microscopic dimensions and high specific surface areas, thermal equilibrium between the droplet and the environment is quickly reached. This situation allows for accomplishing nucleation and freezing of the droplets in a span of milliseconds or seconds [58]. Rapid freezing prevents the damaging effects of phase separation on biomolecular structure by preventing, or at least minimizing, crystallization of excipients and also minimizing solutes partitioning and pH change [73–75]. Figure 1 depicts possible methods for performing the spray freezing step.
Figure 1. Schematic diagram of the spray freeze-drying process.
3.1. Spray Freezing into Vapor (SFV)
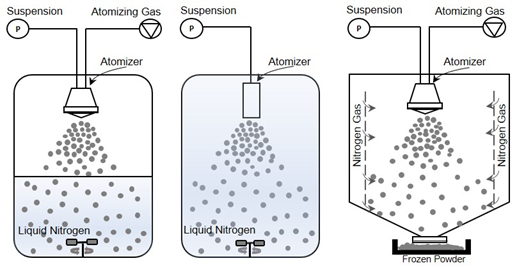
In this configuration, the atomization of a feed solution occurs in a chamber containing a cold vapor (Figure 2-right) [31]. Mumenthaler and Leuenberger [13] proposed spray freezing into the cold gas stream (air in their case) for the purpose of freezing and, then, drying the frozen particles in a fluidized bed at atmospheric pressure. Cold gas passes through the drying chamber for sublimation of frozen solvents and transports the vapor into a recycling chamber. After condensing on a cooled surface, the recycled gas re-enters the chamber for further sublimation. In the case of food products, improved aroma retention, fine powders, free-flowing material, and shorter drying times were achieved compared to the traditional freeze-drying methods. In this configuration, since the counter-current flow is used, collection efficiency and particle elutriation are major concerns. These difficulties were also evidenced by Leuenberger’s [16] group when they successfully obtained an instant water-soluble drug (10–30 µm size) using the SFV technique at atmospheric pressure, capturing the particles with a gas filter. To overcome the above mentioned difficulties, Wang et al. [36] developed a co-current flow process to convey the frozen powder to the exit filter. In this case, cooled nitrogen gas was fed from lateral porous walls into the chamber, where frozen particles are formed and then dried on an exit filter disc at atmospheric pressure. They obtained free-flowing porous powders, preserving the relevant biological properties of BSA and skim milk formulations.
Figure 2. Illustration of different spray freezing techniques. (left) Spray freezing into vapor over liquid, (middle) Spray freezing into liquid, (right) Spray freezing into vapor.
In another approach [31], a liquid solution is sprayed through a piezoelectric droplet generator nozzle into a very cold environment, a jacketed system cooled by liquid nitrogen, and subsequently dried by sublimation. The principle of this process allows to avoid direct contact between the product and liquid or vapor nitrogen, and to easily remove the product after spray freezing. The researchers tested lysozyme and stabilizers (BSA, polyvinylpyrrolidone or dextran) in various formulations and observed free-flowing and porous particles with a diameter ranging between 231 ± 3 µm and 310 ± 10 µm depending on the composition. In addition, enzyme activity was maintained throughout the process for all formulations.
3.2. Spray Freezing into Liquid (SFL)
Another possible method, developed and patented by the University of Texas at Austin in 2001 and commercialized by Dow Chemical Company [76,77], is SFL (Figure 2-middle) in which the insulated nozzle is positioned directly into a cryogenic liquid to produce frozen microparticles [38]. Then the frozen particles are collected and lyophilized [73,78]. During the SFL process, the cryogenic liquid may be stirred by an impeller to prevent clumping of the frozen particles. This method results in ultra-rapid freezing rates and intense atomization where tiny droplets can be obtained due to the higher viscosity and density of a liquid compared to gas and high velocity through an orifice nozzle [47]. This also means that phase separation and pH changes of dissolved substances and crystalline growth of water can be minimized [37,40]. Some studies have evidenced that rapid cooling rates can favor the formation of an amorphous glass [40,79,80], while others concluded that increased freezing rate and ice water interface area increase protein and peptide denaturation [73].
During the SFL process, generally, a capillary nozzle made from polyether–ether ketone (PEEK) characterized by very low thermal conduction is used to spray the liquid formulation because of the risk of clogging of the nozzle by ice formation. Alternatively, the liquid solution may be sprayed into a cryogen through a heated nozzle. However, this may further contribute to the evaporation of cryogenic fluid gases, which will act as an insulating layer around the droplets, strengthening the Leidenfrost effect, which gives more time to form ice crystals due to low thermal conductivity, resulting in low freezing rate [50,72]. It should be noted that when this technique is applied to water-soluble peptides and proteins, no organic solvent or high temperature is used [73].
Rogers et al. [40] compared SFL and slow freezing of danazol formulations in the presence of hydroxypropyl-β-cyclodextrin (HPbCD) for enhanced dissolution. The morphology of the micronized powder obtained from SFL has been found to be better in terms of reconstruction time compared to that obtained by slow freezing. The diameter of the SFL particles was estimated to be approximately 7 µm. The specific surface area of the SFL particles (113.50 m2/g) was significantly larger than that obtained by slow freezing (0.17 m2/g). Due to its high surface area, SFL danazol particles exhibited the best dissolution profiles. In another SFL investigation of danazol formulations containing danazol/polyvinylpyrrolidone (PVP) K-15, danazol/PVP K-15/poloxamer 407, and danazol/PVP K-15/sodium lauryl sulphate, the surface area of the micronized powders was found to be 79.9 m2/g, 30.0 m2/g, and 48.0 m2/g, respectively. Although these powders have different specific surface areas, each of them exhibited enhanced dissolution rates compared to the micronized crystalline danazol [81].
3.3. Spray Freezing into Vapor over Liquid (SFV/L)
The method most employed for spray freezing (Figure 2-left) in pharmaceutical applications is defined as the atomization of a feed solution into a cryogenic vapor over liquid. In the process, the droplet sprayed from a nozzle positioned at a distance above the boiling cryogenic liquid that is stirred or not stirred begins to freeze slowly while falling down through the cold vapor phase before it comes into contact with the cryogen [82]. When they pass through the cold vapor phase, the droplets begin to freeze and become completely frozen as contact is made with the liquid cryogen. Finally, the suspended frozen particles are captured by sieving, or by letting the cryogen evaporate and then transferring them to a freeze-dryer to obtain a dry powder [6].
Yu et al. [83] used SFV/L and SFL to produce lysozyme powders in order to investigate the morphology and stability of powders. The specific surface area of the SFV/L and SFL samples ranged between 25 and 90 m2/g, and was slightly smaller in SFL particles compared to the particles produced by SFV/L. The particle morphology and particle size observed (via SEM imaging) for SFV/L and SFL indicated similar results and particles varied between 100–300 nm size depending on the formulation. In another research, the particle size distribution for seven types of hydrophobic amino acid (L-alanine, glycine, L-isoleucine, L-leucine, L-phenylalanine, L-tryptophan, or L-valine) obtained by SFV/L, varied again with formulation, but the particle diameters ranged between 5 to 10 µm [84]
This entry is adapted from the peer-reviewed paper 10.3390/pr8060709