Ticks are hematophagous ectoparasites that infest a diverse number of vertebrate hosts. The tick immunobiology plays a significant role in establishing and transmitting many pathogens to their hosts. To control tick infestations, the acaricide application is a commonly used method with severe environmental consequences and the selection of tick-resistant populations.
- anti-tick vaccines
- tick immune system
- extracellular traps
- Extracellular Traps Formation (ETosis)
- Neutrophils Extracellular Traps Formation (NETosis)
- cattle diseases
1. Introduction
Direct damages caused by tick bites include blood loss through engorgement, stress, anemia, inflammation, depression of immune function, and allergies caused by antigens and coagulants of the tick saliva [1]. Additionally, ticks’ saliva contains several protein families that induce toxicoses, including paralysis, possibly induced by blocking ion channels that regulate the cell membrane potential in tissues [2].
Ticks are efficient vectors and reservoirs of pathogens, including viruses, bacteria, and protozoans, transmitted to vertebrate hosts [3]. Over time, ticks and ticks-transmitted parasites have co-evolved with their hosts as part of the ecological niche’s equilibrium [4].
On the other hand, the indiscriminate use of chemical acaricides has allowed the selection of resistant populations of ticks and constant environmental pollution [5]. Since the first reports of resistance development of Rhipicephalus spp. to chemical compounds, this ability of ticks has evolved, and now they are resistant to almost all the available acaricides [6][7]. In this regard, the search for new tick control measures is a priority to cope with the adverse impacts of ticks and TBDs on cattle.
So far, the information about the interaction pathogen-vector and the plasticity of ticks’ immune system in response to pathogens is scarce. This review presents a general view of ticks’ immune system and the main molecules that participate in its defense. Here, we propose that discovering new mechanisms in ticks, such as the production of extracellular traps (ETosis), also provides alternatives to identify new targets to develop strategies to control ticks and TBDs. Additionally, the study of the tick immune system components demands a new and more integrative vision of the molecules that participates in a basic but evolutionarily well-adapted process.
2. The Tick Immunobiology
2.1. Tick Signaling Pathways in Immune Response
2.1.1. Toll Pathway
2.1.2. Immune Deficiency (IMD) Pathway
2.1.3. Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) Pathway
2.1.4. RNA Interference (RNAi) Pathway
2.2. The Cellular Immune Response
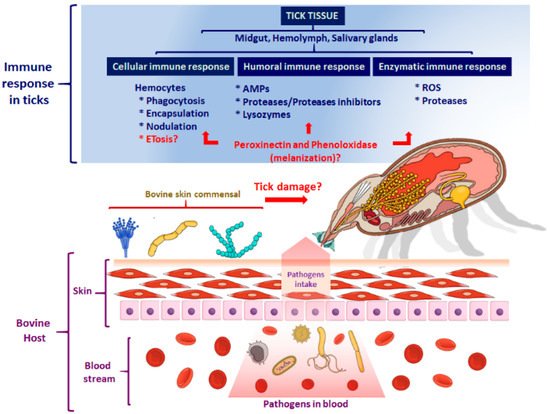
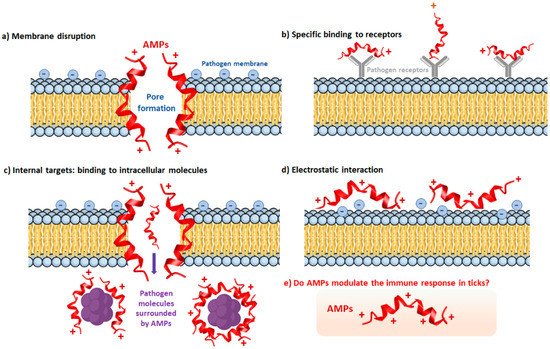
3. The Humoral Immune Response of Ticks
The Tick Repertoire of Humoral Factors
This entry is adapted from the peer-reviewed paper 10.3390/pathogens10111511
References
- Ghosh, S.; Azhahianambi, P.; Yadav, M.P. Upcoming and future strategies of tick control: A review. J. Vector Borne Dis. 2007, 44, 79–89.
- Maritz, C.; Louw, A.; Gothe, R.; Neitz, A. Neuropathogenic properties of Argas (Persicargas) walkerae larval homogenates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 233–239.
- Hajdušek, O.; Šíma, R.; Ayllón, N.; Jalovecká, M.; Perner, J.; De La Fuente, J.; Kopáček, P. Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 2013, 3, 26.
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14.
- Kunz, S.; Kemp, D. Insecticides and acaricides: Resistance and environmental impact. Rev. Sci. Tech. 1994, 13, 1249–1286.
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20.
- George, J.E.; Pound, J.M.; Davey, R.B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129, S353–S366.
- Kvell, K.; Cooper, E.; Engelmann, P.; Bovari, J.; Németh, P. Blurring borders: Innate immunity with adaptive features. Clin. Dev. Immunol. 2007, 83671.
- Fogaça, A.C.; Sousa, G.; Pavanelo, D.B.; Esteves, E.; Martins, L.A.; Urbanova, V.; Kopacek, P.; Daffre, S. Tick immune system: What is known, the interconnections, the gaps, and the challenges. Front. Immunol. 2021, 12, 119.
- Guerrero, F.; Miller, R.; Rousseau, M.-E.; Sunkara, S.; Quackenbush, J.; Lee, Y.; Nene, V. BmiGI: A database of cDNAs expressed in Boophilus microplus, the tropical/southern cattle tick. Insect Biochem. Mol. Biol. 2005, 35, 585–595.
- Megy, K.; Emrich, S.J.; Lawson, D.; Campbell, D.; Dialynas, E.; Hughes, D.S.; Koscielny, G.; Louis, C.; MacCallum, R.; Redmond, S.; et al. VectorBase: Improvements to a bioinformatics resource for invertebrate vector genomics. Nucleic Acids Res. 2012, 40, D729–D734.
- Gulia-Nuss, M.; Nuss, A.; Meyer, J.M.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; De La Fuente, J.; Ribeiro, J.; Megy, K.; et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507.
- Rosa, R.D.; Peixoto, J.; Mesquita, R.D.; Kalil, S.P.; Pohl, P.C.; Braz, G.R.; Fogaça, A.; Daffre, S. Exploring the immune signalling pathway-related genes of the cattle tick Rhipicephalus microplus: From molecular characterization to transcriptional profile upon microbial challenge. Dev. Comp. Immunol. 2016, 59, 1–14.
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti Toll pathway controls dengue virus infection. PLoS Pathog. 2008, 4, e1000098.
- Lemaitre, B. The road to Toll. Nat. Rev. Immunol. 2004, 4, 521–527.
- Kleino, A.; Silverman, N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 2014, 42, 25–35.
- Palmer, W.J.; Jiggins, F.M. Comparative genomics reveals the origins and diversity of arthropod immune systems. Mol. Biol. Evol. 2015, 32, 2111–2129.
- Narasimhan, S.; Schuijt, T.J.; Abraham, N.M.; Rajeevan, N.; Coumou, J.; Graham, M.; Robson, A.; Wu, M.-J.; Daffre, S.; Hovius, J.W.; et al. Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun. 2017, 8, 184.
- Tafesh-Edwards, G.; Eleftherianos, I. JNK signaling in Drosophila immunity and homeostasis. Immunol. Lett. 2020, 226, 7–11.
- Shaw, D.K.; Wang, X.; Brown, L.J.; Chávez, A.S.O.; Reif, K.E.; Smith, A.A.; Scott, A.J.; McClure, E.E.; Boradia, V.M.; Hammond, H.L.; et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 2017, 8, 14401.
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.-L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953.
- Liu, L.; Dai, J.; Zhao, Y.O.; Narasimhan, S.; Yang, Y.; Zhang, L.; Fikrig, E. Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J. Infect. Dis. 2012, 206, 1233–1241.
- Shuai, K.; Ziemiecki, A.; Wilks, A.F.; Harpur, A.G.; Sadowski, H.B.; Gilman, M.Z.; Darnell, J.E. Polypeptide signaling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nat. Cell Biol. 1993, 366, 580–583.
- Capelli-Peixoto, J.; Carvalho, D.D.; Johnson, W.C.; Scoles, G.A.; Fogaça, A.C.; Daffre, S.; Ueti, M.W. The transcription factor Relish controls Anaplasma marginale infection in the bovine tick Rhipicephalus microplus. Dev. Comp. Immunol. 2017, 74, 32–39.
- Karlikow, M.; Goic, B.; Saleh, M.-C. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Dev. Comp. Immunol. 2014, 42, 85–92.
- Schnettler, E.; Tykalová, H.; Watson, M.; Sharma, M.; Sterken, M.; Obbard, D.; Lewis, S.H.; McFarlane, M.; Bell-Sakyi, L.; Barry, G.; et al. Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res. 2014, 42, 9436–9446.
- Blair, C.D. Mosquito RNAi is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011, 6, 265–277.
- Asgari, S. Role of microRNAs in arbovirus/vector interactions. Viruses 2014, 6, 3514–3534.
- Budachetri, K.; Karim, S. An insight into the functional role of thioredoxin reductase, a selenoprotein, in maintaining normal native microbiota in the Gulf Coast tick (Amblyomma maculatum). Insect Mol. Biol. 2015, 24, 570–581.
- Narasimhan, S.; Sukumaran, B.; Bozdogan, U.; Thomas, V.; Liang, X.; DePonte, K.; Marcantonio, N.; Koski, R.A.; Anderson, J.F.; Kantor, F.; et al. A tick antioxidant facilitates the Lyme disease agent’s successful migration from the mammalian host to the arthropod vector. Cell Host Microbe 2007, 2, 7–18.
- Paradkar, P.N.; Duchemin, J.-B.; Voysey, R.; Walker, P.J. Dicer-2-dependent activation of Culex vago occurs via the TRAF-Rel2 signaling pathway. PLoS Negl. Trop. Dis. 2014, 8, e2823.
- Lässer, C. Exosomes in diagnostic and therapeutic applications: Biomarker, vaccine and RNA interference delivery vehicle. Expert Opin. Biol. Ther. 2015, 15, 103–117.
- Sonenshine, D.E.; Hynes, W.L. Molecular characterization and related aspects of the innate immune response in ticks. Front. Biosci. 2008, 1, 7046–7063.
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14.
- Urbanová, V.; Šíma, R.; Sauman, I.; Hajdusek, O.; Kopáček, P. Thioester-containing proteins of the tick Ixodes ricinus: Gene expression, response to microbial challenge and their role in phagocytosis of the yeast Candida albicans. Dev. Comp. Immunol. 2015, 48, 55–64.
- Eggenberger, L.R.; Lamoreaux, W.J.; Coons, L.B. Hemocytic encapsulation of implants in the tick Dermacentor variabilis. Exp. Appl. Acarol. 1990, 9, 279–287.
- Ceraul, S.M.; Sonenshine, D.E.; Hynes, W. Resistance of the tick Dermacentor variabilis (Acari: Ixodidae) following challenge with the bacterium Escherichia coli (Enterobacteriales: Enterobacteriaceae). J. Med. Entomol. 2002, 39, 376–383.
- Zhang, L.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19.
- Fogaça, A.C.; Lorenzini, D.M.; Kaku, L.M.; Esteves, E.; Bulet, P.; Daffre, S. Cysteine-rich antimicrobial peptides of the cattle tick Boophilus microplus: Isolation, structural characterization and tissue expression profile. Dev. Comp. Immunol. 2004, 28, 191–200.
- Kopácek, P.; Hajdusek, O.; Buresová, V.; Daffre, S. Tick innate immunity. Adv. Exp. Med. Biol. 2010, 708, 137–162.
- Taylor, D. Innate Immunity in Ticks: A review. J. Acarol. Soc. Jpn. 2006, 15, 109–127.
- Cabezas-Cruz, A.; Tonk, M.; Bleackley, M.R.; Valdés, J.J.; Barrero, R.A.; Hernández-Jarguín, A.; Moutailler, S.; Vilcinskas, A.; Richard-Forget, F.; Anderson, M.A.; et al. Antibacterial and antifungal activity of defensins from the Australian paralysis tick, Ixodes holocyclus. Ticks Tick-Borne Dis. 2019, 10, 101269.
- Nakajima, Y.; Naters-Yasui, A.V.D.G.V.; Taylor, D.; Yamakawa, M. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol. Biol. 2002, 11, 611–618.
- Janeway, C.A.J.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216.
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236.
- Valenzuela, J.G.; Francischetti, I.M.B.; Pham, V.M.; Garfield, M.K.; Mather, T.N.; Ribeiro, J.M.C. Exploring the sialome of the tick Ixodes scapularis. J. Exp. Biol. 2002, 205, 2843–2864.
- Blisnick, A.A.; Foulon, T.; Bonnet, S.I. Serine protease inhibitors in ticks: An overview of their role in tick biology and tick-borne pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 199.
- Saravanan, T.; Weise, C.; Sojka, D.; Kopáček, P. Molecular cloning, structure and bait region splice variants of α2-macroglobulin from the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 2003, 33, 841–851.
