Ursolic acid (UA) is a promising triterpenoid compound present in several plants’ leaves, flowers, and fruits. It shows a broad range of pharmaceutical properties and therapeutic effects. UA has been utilized as a herbal medicine with excellent pharmacological activities.
- ursolic acid
- diabetic neuropathy
- inflammatory diseases
- inhibitors
- targeted therapy
1. Introduction
Ursolic acid (UA) is a promising triterpenoid compound present in several plants’ leaves, flowers, and fruits [1]. It shows a broad range of pharmaceutical properties and therapeutic effects [2]. UA has been utilized as a herbal medicine with excellent pharmacological activities [3][4]. It mediates alterations in many signaling pathways and subsequently prevents the progression of chronic diseases [5], demonstrating its anti-inflammatory [6], anti-oxidant [7], anti-carcinogenic [8], anti-diabetic [9], neuroprotective effects [10]. UA is effective in the treatment of many inflammatory diseases [4], including Parkinson’s disease [11] and diabetes [12]. In addition, UA has been implicated in neurodegenerative and psychiatric disorders [13]. It has considerable potential as an oral anti-inflammatory and neural repair drug [14], attenuating neuropathic pain in animal models that can probably be attributed to its anti-oxidant and anti-inflammatory properties [15]. The mechanisms of UA by which applies these beneficial consequences might engage regulation of metabolic and atrophy pathway in skeletal muscles, insulin pathway in adipose tissue, apoptotic and NF-κB pathway in tumor cells, level of oxidants and metabolic pathway in the liver, anti-oxidants and inflammation in the brain [16][17][18] .
Cancer is the leading cause of human death in economically developed countries [19][20][21]. The cancer burden keeps on enhancing in developing countries due to increasing aging in the population [21][22]; cancer is a major cause of adult deaths worldwide [21][23]. Cancer is a complex disease that may be inhibited [24][25]; cancer progression might take around 10 to 30 years to develop, starting initiation, promotion for development, and progression [24][25]. Hence, the slow growth of the disease potentially permits interference in the development of tumors into advanced stages and metastases. This disease has a high incidence and mortality rate; its treatment indicates a significant clinical challenge. Hence, the advantages of present chemotherapeutics are inadequate because they tend to cause DNA damage in healthy cells [26][27]. However, targeting the regulation of cancer cells without causing toxic effects in healthy cells is a potential strategy for cancer treatment [28][29].
Inflammation engages the activation and employment of phagocytes (neutrophils, macrophages), NK cells, and the discharge of cytokines through activated cells that are crucial to the host defense system. Since the chronic inflammation that continues yet after removal of the pathogen(s) was linked with many diseases, including cancer [30], neoplasms, inflammatory bowel disease [31], Alzheimer’s disease [32], therefore, dysregulation of enzymes and cytokines, might contribute to the pathogenesis of several chronic inflammatory diseases [33]. NF-κB , NF-AT, and AP1 with JNK, ERK, and p38 are well-known for regulating inflammatory cytokines and enzymes targeted through various investigators to improve chronic inflammation [34][35][36]. Inflammation plays a fundamental role in the pathogenesis of DN [37].
The complexities of DN and inflammatory diseases have considerably evolved over the years. Efforts for understanding the etiology of DN and inflammatory diseases are in progress to design attractive therapeutic approaches. In this context, UA may be a potent therapeutic molecule for DN and inflammatory diseases. UA and its derivatives are potential therapeutic candidates that can be explored to treat DN and inflammatory diseases. In this review, we systematically collected current findings concerning biochemistry, potency, pharmacological aspect, drug delivery system, and clinical trials of UA.
2. Biological Potency of UA
The molecular action of bioactive molecules might open up novel opportunities to the scientific community for developing and improving new therapeutic approaches for tackling dreadful diseases, including neurodegenerative disorders. UA is one such plant-based therapeutic metabolite, which plays a vital function in cell death, angiogenesis, metastasis, and inflammatory processes [38]. Table 1 presents various health benefits, including anti-apoptotic, anti-oxidant, anti-inflammatory, anti-carcinogenic, anti-rheumatic, anti-tumoral, anti-viral, trypanocidal, etc. , of UA [39][40]. UA has anti-cancer activity due to low toxicity and commercial accessibility forms [41][42].
| Disease | Experimental Subject | Dosage | Beneficial Effects | References |
|---|---|---|---|---|
| Diabetes | 3T3-L1 adipocytes | 1 μg/mL for 10 min | ↑ Akt, insulin receptor, and GLUT 4 ↑ Glycogen synthase kinase-3β |
[43] |
| Diabetes | Streptozotocin-injected male ICR mice |
0.5 g/kg for 4 weeks | ↓ TNF-α and Glucose ↑ Insulin (pancreatic, plasma) |
[44] |
| Diabetes | Streptozotocin-injected male mice |
200 mg/kg per day for 6 weeks |
↓ Adipocyte dysfunction ↓ Fasting blood glucose ↓ PPAR γ and aP2 ↑ Bone formation |
[45] |
| Metabolic syndrome | Diagnostics of metabolic syndrome patients |
Orally 150 mg/kg for 12 weeks | ↓ Body weight, BMI, and waist circumference ↓ Fasting glucose |
[46] |
| Subarachnoid hemorrhage (SAH) |
Male Sprague Dawley experimental SAH rat model |
25 and 50 mg/kg at 0.5, 24, and 47 h after SAH |
↓ MDA ↑ Neurological score ↑ Cerebral vasospasm ↓ BBB permeability (EB content) ↑ GSH/GSSH ratio, SOD activity, and Catalase activity ↓ Apoptotic index ↓ Caspase-3, -9 mRNA expression |
[47] |
| Parkinson’s disease | Male Swiss albino mice |
5, 25, and 50 mg/kg for 21 days |
↑ Rotarod test ↑ Hanging time ↓ Nitrite level ↓ Narrow beam walking test ↑Acidhomovanilic acid ↑ Dopamine |
[48] |
| Cerebral ischemia and reperfusion injury |
Male Sprague Dawley rats |
5, 10, and 20 mg/kg at 0.5, 24, and 47 h after reperfusion |
↓ Neurological deficit score ↓ Infarct volume ↑ PPARγ protein level ↑ Number of intact neurons ↓MMP-2 & -9 protein levels |
[49] |
| IL-1β or TNF-α- induced C6 glioma invasion |
Rat C6 glioma cells | 5, 10, and 20 μM for 24 h |
↓ MMP-9 activity by TNF-α or IL-1β ↓ IκB kinase activity by IL-1β or TNF-α ↓ IκBα activity by IL-1β or TNF-α ↓ NF-κB activity |
[50] |
| D-Galactose-induced neurodegenerative changes |
Male Kunming strain mice |
10 mg/kg for 8 weeks |
↓ ROS level ↓ AGEs level ↓ Number of CD11b-stained cells, ↓ Carbonyl protein level GFAPstained cells, and RAGE- positive cells ↓ iNOS, IL-6, IL-1β, COX-2, and TNF-α protein levels |
[51] |
| Domoic acid-induced cognitive deficits |
Male ICR mice | 100 mg/kg for 3 weeks | ↑p-Akt ↑ HO-1 ↑ p-FOXO1 ↑ Complex I-V ↑ Electron transport chain activity ↑ APR and ATP |
[52] |
| Adrenocorticotrophic hormone-producing pituitary adenoma |
AtT20 cells (mouse corticotrophic tumor cell line) |
10, 20, and 40 μM for 24 h |
↓ ACTH release ↓ POMC mRNA expression ↓ ACTH protein level ↑ p-JNK/JNK protein level |
[53] |
↓, Decrease; ↑, Increase.
UA is popular due to its anti-proliferative properties, the incentive of tumor cell death, an obstacle of tumorigenesis, and inhibiting the cell cycle in tumors cells. Hence, examining one cell death mechanism that indicated UA could prevent NF-κB pathway through p65 phosphorylation repression, effecting a mandatory reduction in several downstream oncogenes, including Bcl-xL and Bcl-2. The anti-cancer effectiveness of UA is weak due to its lesser solubility that reduces the drug absorption in the human body system, which causes challenges in achieving comprehensive benefits. However, it is required for making its derivatives by semi-synthetic alterations for improving its anti-cancer action. Frequently, the modifications of UA occur on positions C-3, C12–C13, and positions C-28 [54][55][56]. Antimicrobial activity of UA compounds against Mycobacterium tuberculosis H37Rv has been recently reported [57][58]. UA is currently in different phases of clinical studies due to its therapeutic effects and selectivity against several diseases [59][60].
3. Bioavailability and Pharmacokinetic Properties of UA
Cell membrane permeability and pharmacokinetics are vital in the clinical development and improvement of novel biologically active agents/compounds with an outlook for considering their performance in vivo and establishing the most acceptable dosage regimen. Hence, pentacyclic triterpenoids usually suffer from less oral bioavailability [61]. In the organism, a vast inter and intra character absorption variability shows challenges in accomplishing secure and efficient concentration of drug [62]. Physicochemical characters of the molecules are the first reason which affects bioavailability [63]. UA is a low molecular weight compound [64], with three hydrogen bond donors and acceptors. However, these properties for estimating the drug-likeness are in harmony with Lipinski’s rule [65]. UA has more lipophilicity [61] and less wettability [66]. Hence, their absorption is obstructed through slow partitioning between extracellular fluid and cell membrane and poor dissolution. UA can be inserted in the phospholipid bilayer while not taken up via cells [66].
Moreover, aqueous solubility is exaggerated through the crystalline structure of natural UA. Decreased particle size and amorphous state remarkably increased rate of dissolution and solubility of triterpenoid [67]. Adverse characteristics of UA should be addressed in the improvement of pharmaceutical dose shapes. Hence, for overcoming biological barriers, the second main factor is the capability of the drug [63]. The proof from in vitro permeability investigations involves which passive diffusion is the primary process of UA transport [63]. Hence, apparent permeability coefficients, estimated applying Caco-2 monolayers, have been in limits of oral absorption [63], which instant glucuronidation, and sulfation in the intestinal cells, are extremely unlikely [68]. UA is a substrate of cytochrome P450 and P-glycoprotein. Therefore their bio-availability can be limited through biotransformation and active efflux [67][69].
Many pharmacokinetic examinations have exposed where maximal plasma concentration subsequent oral administration of doses up to 300 mg/kg has been low, and removal half-life has been comparatively short (<1 h) [70][67]. This pharmacokinetic outline shows that fast elimination and tissue distribution in the pharmacological results of UA may not be honestly related to plasma concentrations. UA has broad tissue distribution, such as in the animal’s testes, colon, lung, kidney, spleen, brain, liver, heart and bladder [70][71]. The liver is the main organ of the triterpene disposition [71], and liver-related doses limit the toxicity in clinical trial phase I of UA liposomes [72]. UA can cross the blood-brain barrier, and they have potent neuroprotective effects [71]. Targeted delivery for the brain may be attained through particular delivery systems that use UA nano-lipid vesicles in the form of intranasal gel [73]. Hence, high lipophilicity influences triterpenoids for liver metabolism. However, a tissue distribution examination in mice has shown that the UA concentration in plasma was gradually reduced, but the concentration in the liver increased [74].
4. Pharmacological Aspects of UA
In cerebral ischemia and reperfusion injury, TLRs play a critical role by inducing the making of inflammatory mediators, including ILs and TNF-α [75][76][77]. TLR4 was primarily reported as receptors to endogenous ligands such as DAMPs, and HMGB1, during brain injury. UA controls the TLR pathway and shows prominent anti-inflammatory functions. UA demonstrates biological actions in the brain, such as anti-inflammatory, anti-oxidative, anti-rheumatic and anti-tumor effects [78]. UA decreases inflammatory cytokine-making to protect the brain from cerebral ischemia and reperfusion injury, probably by HMGB1/TLR4/NF-κB pathway [79]. UA might be helpful as a potential efficient adjunct for therapy to ischemic brain injury before reperfusion.
Inflammation plays an essential role in developing and progressing multiple diseases, including neuropathy, insulin resistance, and diabetes [80], cancer [81][82][83][84][85]. After injury and microbial attack, the inflammatory reaction is started to recover homeostatic tissue equilibrium between composition and physiological role. Hence, persistent inflammation can cause harm to tissues, affecting non-functioning tissues/organs [86][87]. Inflammation is an obscured incidence connected to the progression of various diseases, including neurodegenerative diseases and cancer [88]. Acute inflammation, with attendant cytokine action and the increased output of ROS, is reported like a tumor-promoting disease [89][90][91]. For detecting the anti-inflammatory activity, reported UA’s capability to reduce the making of TNF-α in A549 and RAW 267.4 cell lines infected by Mycobacterium tuberculosis and Con A-stimulated mouse splenocytes.
The authors examined UA action intending to reduce COX-2 and NO synthase levels found in roused cells. Hence, UA showed a considerable inhibitory effect of cytokine levels, immunomodulatory mediators, and liberate of NO. This compound may be used for tuberculosis and antibiotic therapy due to the UA’s anti-inflammatory effectiveness in cells [92]. It showed in vitro inhibition of COX-2 action was because of UA and cranberry extracts [93], indicating potential anti-inflammatory and anti-oxidant activity ( Table 2 ). The anti-inflammatory activity was investigated by utilizing enzyme inhibitory examine in vitro COX-1 and COX-2 [94][95]. The molecular docking discoveries exhibited; the UA derivatives indicated elevated attraction to effective COX-2 site, potentially showing anti-inflammatory effectiveness by COX-2 inhibition. Hence, UA and its derivatives showed anti-inflammatory activity that might cause the development and improvement of potentially novel and secure COX-2 inhibitors [96][97]. The anti-inflammatory mechanisms inhibit main inflammatory cytokines, iNOS and COX expressions, and anti-oxidant mechanisms like the activation of Nrf2 signaling. The inflammatory and anti-oxidant mechanisms of neuroprotection through UA [98] are also indicated by the plethora of extra systemic results of UA in several experimental models [98]. The effects were linked with the inhibition of NF-κB translocation to the nucleus ( Figure 1 ) and decreased expression of iNOS, COX-2, TNF-α, and interleukin-6 that decreased the phosphorylation of p38MAPK in the mouse brain [99].
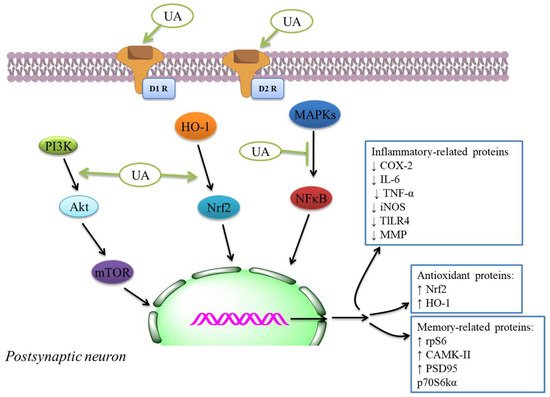
Terpenoids could have anti-oxidant results in vivo through mediating anti-oxidant defenses, including CAT, SOD, GPx, and GR, exhibited to their therapeutic potent in AD by a range of assays [100][101][102][103][104]. These compounds display anti-oxidant effects and possess anti-inflammatory functions. They are tackling complex diseases by what has been explained as one drug → multi-targets → one/many disease(s) therapeutic theory [105]. In this perspective, the therapeutic promising of UA like a prototype lead is exhibited in several CNS diseases, mainly by anti-inflammatory and anti-oxidant mechanisms. The anti-oxidant–anti-inflammatory axis was indicated to play a function in the anti-diabetic effect of UA as displayed in streptozotocin-mediated rats [106] in db/db diabetic mouse model [107] , DN models [108], diabetic-mediated monocyte dysfunction in mice [109], aortic damage in the STZ-mediated diabetic rats [110].
This entry is adapted from the peer-reviewed paper 10.3390/ijms222212162
References
- Jager, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031.
- Wozniak, L.; Skapska, S.; Marszalek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641.
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmad, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012–2016). Expert Opin. Ther. Patents. 2017, 27, 1061–1072.
- Lee, S.Y.; Kim, Y.J.; Chung, S.O.; Park, S.U. Recent studies on ursolic acid and its biological and pharmacological activity. EXCLI J. 2016, 15, 221.
- Van Kiem, P.; Hang, D.T.; Nhiem, N.X.; Tai, B.H.; Anh, H.L.T.; Van Cuong, P.; Quang, T.H.; Van Minh, C.; Van Dau, N.; Kim, Y.A.; et al. Sesquiterpene derivatives from marine sponge Smenospongia cerebriformis and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2017, 27, 1525–1529.
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Punia, S.; Sharma, A.K. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent. Pat. Inflamm. Allergy. Drug. Discov. 2016, 10, 21–33.
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644.
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383.
- Yu, S.G.; Zhang, C.J.; Xu, X.E.; Sun, J.H.; Zhang, L.; Yu, P.F. Ursolic acid derivative ameliorates streptozotocin-induced diabestic bone deleterious effects in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 3681–3690.
- Wang, Y.; He, Z.; Deng, S. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug. Des. Devel. Ther. 2016, 10, 1663–1674.
- Mortiboys, H.; Aasly, J.; Bandmann, O. Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain 2013, 136, 3038–3050.
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931.
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 2017, 31, 1029–1041.
- Zhang, Y.; Li, X.; Ciric, B.; Curtis, M.T.; Chen, W.-J.; Rostami, A.; Zhang, G.-X. A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc. Natl. Acad. Sci. USA 2020, 117, 9082–9093.
- Bhat, R.A.; Lingaraju, M.C.; Pathak, N.N.; Kalra, J.; Kumar, D.; Kumar, D.; Tandan, S.K. Effect of ursolic acid in attenuating chronic constriction injury-induced neuropathic pain in rats. Fundam. Clin. Pharmacol. 2016, 30, 517–528.
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248.
- Dhakal, H.; Kim, M.J.; Lee, S.; Choi, Y.A.; Kim, N.; Kwon, T.K.; Khang, D.; Kim, S.-H. Ursolic acid inhibits FcepsilonRI-mediated mast cell activation and allergic inflammation. Int. Immunopharmacol. 2021, 99, 107994.
- Son, J.; Lee, S.Y. Therapeutic Potential of Ursonic Acid: Comparison with Ursolic Acid. Biomolecules 2020, 10, 1505.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2018, 68, 394–424.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917.
- Alam, M.; Kashyap, T.; Pramanik, K.K.; Singh, A.K.; Nagini, S.; Mishra, R. The elevated activation of NFκB and AP-1 is correlated with differential regulation of Bcl-2 and associated with oral squamous cell carcinoma progression and resistance. Clin. Oral Investig. 2017, 21, 2721–2731.
- Alam, M.; Kashyap, T.; Mishra, P.; Panda, A.K.; Nagini, S.; Mishra, R. Role and regulation of proapoptotic Bax in oral squamous cell carcinoma and drug resistance. Head Neck 2019, 41, 185–197.
- Sporn, M.B. Perspective: The big C—For Chemoprevention. Nature 2011, 471, S10–S11.
- Alam, M.; Mishra, R. Bcl-xL expression and regulation in the progression, recurrence, and cisplatin resistance of oral cancer. Life. Sci. 2021, 280, 119705.
- Ramirez, C.N.; Li, W.; Zhang, C.; Wu, R.; Su, S.; Wang, C.; Gao, L.; Yin, R.; Kong, A.-N. In Vitro-In Vivo Dose Response of Ursolic Acid, Sulforaphane, PEITC, and Curcumin in Cancer Prevention. AAPS J. 2017, 20, 19.
- Rana, R.; Rathi, V.; Chauhan, K.; Jain, K.; Chhabra, S.S.; Acharya, R.; Kalra, S.K.; Gupta, A.; Jain, S.; Ganguly, K.N.; et al. Exploring the role of epidermal growth factor receptor variant III in meningeal tumors. PLoS ONE 2021, 16, e0255133.
- Li, Y.; Wang, Y.; Zhou, Y.; Li, J.; Chen, K.; Zhang, L.; Deng, M.; Deng, S.; Li, P.; Xu, B. Cooperative effect of chidamide and chemotherapeutic drugs induce apoptosis by DNA damage accumulation and repair defects in acute myeloid leukemia stem and progenitor cells. Clin. Epigenet. 2017, 9, 1–14.
- Ali, S.; Alam, M.; Hasan, G.M.; Hassan, M.I. Potential therapeutic targets of Klebsiella pneumoniae: A multi-omics review perspective. Brief. Funct. Genom. 2021, elab038.
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 1–18.
- Singh, D.D.; Verma, R.; Parimoo, P.; Sahu, A.; Kumar, V.; Upadhyay, E.; Yadav, D.K. Potential Therapeutic Relevance of CRISPR/Cas9 Guided Epigenetic Regulations for Neuropsychiatric Disorders. Curr. Top. Med. Chem. 2021, 21, 878–894.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Endoplasmic reticulum stress: Implications for inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. Annu. Rev. Immunol. 2010, 28, 573–621.
- Lee, Y.-J.; Han, S.B.; Nam, S.-Y.; Oh, K.-W.; Hong, J.T. Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 2010, 33, 1539–1556.
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45.
- Sica, A.; Dorman, L.; Viggiano, V.; Cippitelli, M.; Ghosh, P.; Rice, N.; Young, H.A. Interaction of NF-κB and NFAT with the interferon-γ promoter. J. Biol. Chem. 1997, 272, 30412–30420.
- Falvo, J.V.; Uglialoro, A.M.; Brinkman, B.M.N.; Merika, M.; Parekh, B.S.; Tsai, E.Y.; King, H.C.; Morielli, A.D.; Peralta, E.G.; Maniatis, T.; et al. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor alpha gene promoter. Mol. Cell. Biol. 2000, 20, 2239–2247.
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213.
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331.
- Wu, P.-P.; Zhang, B.-J.; Cui, X.-P.; Yang, Y.; Jiang, Z.-Y.; Zhou, Z.-H.; Zhong, Y.-Y.; Mai, Y.-Y.; Ouyang, Z.; Chen, H.-S.; et al. Synthesis and biological evaluation of novel ursolic acid analogues as potential α-glucosidase inhibitors. Sci. Rep. 2017, 7, 1–12.
- Batra, A.; Sastry, V.G. Extraction of ursolic acid from Ocimum sanctum and synthesis of its novel derivatives: Effects on extracellular homocysteine, dihydrofolate reductase activity and proliferation of HepG2 human hepatoma cells. Pteridines 2013, 24, 191–199.
- Stiti, N.M.; Hartmann, M.-A.E. Nonsterol triterpenoids as major constituents of Olea europaea. J. Lipids 2012, 2012, 476595.
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250.
- Jang, S.-M.; Yee, S.-T.; Choi, J.; Choi, M.-S.; Do, G.-M.; Jeon, S.-M.; Yeo, J.; Kim, M.-J.; Seo, K.-I.; Lee, M.-K. Ursolic acid enhances the cellular immune system and pancreatic β-cell function in streptozotocin-induced diabetic mice fed a high-fat diet. Int. Immunopharmacol. 2009, 9, 113–119.
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, 792.
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of ursolic acid on metabolic syndrome, insulin sensitivity, and inflammation. J. Med. Food 2017, 20, 882–886.
- Zhang, T.; Su, J.; Wang, K.; Zhu, T.; Li, X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 2014, 579, 12–17.
- Rai, S.N.; Yadav, S.K.; Singh, D.; Singh, S.P. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J. Chem. Neuroanat. 2016, 71, 41–49.
- Lee, T.H.; Khan, Z.; Subedi, L.; Kim, S.Y.; Lee, K.R. New bis-thioglycosyl-1,1’-disulfides from Nasturtium officinale R. Br. and their anti-neuroinflammatory effect. Bioorg. Chem. 2019, 86, 501–506.
- Huang, H.C.; Huang, C.Y.; Lin-Shiau, S.Y.; Lin, J.K. Ursolic acid inhibits IL-1β or TNF-α-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-ζ and downregulating the MMP-9 expression. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Anderson Cancer Cent. 2009, 48, 517–531.
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Hu, B.; Zhang, Z.-F.; Ye, Q.; Liu, C.-M.; Shan, Q.; Wang, Y.-J. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb. Cortex. 2010, 20, 2540–2548.
- Wu, D.-M.; Lu, J.; Zhang, Y.-Q.; Zheng, Y.-L.; Hu, B.; Cheng, W.; Zhang, Z.-F.; Li, M.-Q. Ursolic acid improves domoic acid-induced cognitive deficits in mice. Toxicol. Appl. Pharmacol. 2013, 271, 127–136.
- Gong, Y.Y.; Liu, Y.Y.; Yu, S.; Zhu, X.N.; Cao, X.P.; Xiao, H.P. Ursolic acid suppresses growth and adrenocorticotrophic hormone secretion in AtT20 cells as a potential agent targeting adrenocorticotrophic hormone-producing pituitary adenoma. Mol. Med. Rep. 2014, 9, 2533–2539.
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655.
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.; Arriaga, Ã.N.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filhoand, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327.
- Tian, T.; Liu, X.; Lee, E.S.; Sun, J.; Feng, Z.; Zhao, L.; Zhao, C. Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anti-cancer agents. Arch. Pharmacal Res. 2017, 40, 458–468.
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complementary Altern. Med. 2013, 13, 1–11.
- Ali, S.; Ehtram, A.; Arora, N.; Manjunath, P.; Roy, D.; Ehtesham, N.Z.; Hasnain, S.E. The M. tuberculosis Rv1523 Methyltransferase Promotes Drug Resistance Through Methylation-Mediated Cell Wall Remodeling and Modulates Macrophages Immune Responses. Front. Cell Infect. Microbiol. 2021, 11, 622487.
- Kataev, V.E.; Khaybullin, R.N.; Garifullin, B.F.; Sharipova, R.R. New Targets for Growth Inhibition of Mycobacterium tuberculosis: Why Do Natural Terpenoids Exhibit Antitubercular Activity? Russ. J. Bioorganic Chem. 2018, 44, 438–452.
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881.
- Jc Furtado, N.A.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400.
- Tong, H.H.Y.; Du, Z.; Wang, G.N.; Chan, H.M.; Chang, Q.; Lai, L.C.M.; Chow, A.H.L.; Zheng, Y. Spray freeze drying with polyvinylpyrrolidone and sodium caprate for improved dissolution and oral bioavailability of oleanolic acid, a BCS Class IV compound. Int. J. Pharm. 2011, 404, 148–158.
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. Bioavailability challenges associated with development of saponins as therapeutic and chemopreventive agents. Curr. Drug Targets 2012, 13, 1885–1899.
- Pironi, A.M.; de Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A review. Crit. Rev. Anal. Chem. 2018, 48, 86–93.
- Kalani, K.; Yadav, D.K.; Khan, F.; Srivastava, S.K.; Suri, N. Pharmacophore, QSAR, and ADME based semisynthesis and in vitro evaluation of ursolic acid analogs for anticancer activity. J. Mol. Modeling 2012, 18, 3389–3413.
- Song, J.; Wang, Y.; Song, Y.; Chan, H.; Bi, C.; Yang, X.; Yan, R.; Wang, Y.; Zheng, Y. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. AAPS PharmSciTech. 2014, 15, 11–19.
- Yang, L.; Sun, Z.; Zu, Y.; Zhao, C.; Sun, X.; Zhang, Z.; Zhang, L. Physicochemical properties and oral bioavailability of ursolic acid nanoparticles using supercritical anti-solvent (SAS) process. Food Chem. 2012, 132, 319–325.
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; McCoy, J.-A.; Widrlechner, M.P.; Reddy, M.B.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112.
- Jinhua, W.; Ying, Z.; Yuhua, L. PXR-ABC drug transporters/CYP-mediated ursolic acid transport and metabolism in vitro and vivo. Arch. Pharm. 2020, 353, 2000082.
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a liquid chromatography—Mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011, 399, 2877–2884.
- Yin, M.-C.; Lin, M.-C.; Mong, M.-C.; Lin, C.-Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701.
- Wang, X.-H.; Zhou, S.-Y.; Qian, Z.-Z.; Zhang, H.-L.; Qiu, L.-H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.-S.; Wang, H.-Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125.
- Khan, K.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Sultana, Y.; Mujeeb, M. Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: Formulation, optimization, in-vivo brain/plasma distribution study and histopathological assessment. Biomed. Pharmacother. 2018, 106, 1578–1585.
- Zhou, X.J.; Hu, X.M.; Yi, Y.M.; Wan, J. Preparation and body distribution of freeze-dried powder of ursolic acid phospholipid nanoparticles. Drug Dev. Ind. Pharm. 2009, 35, 305–310.
- Wu, D.; Lee, Y.-C.G.; Liu, H.-C.; Yuan, R.-Y.; Chiou, H.-Y.; Hung, C.-H.; Hu, C.-J. Identification of TLR downstream pathways in stroke patients. Clin. Biochem. 2013, 46, 1058–1064.
- Guan, J.; Wei, X.; Qin, S.; Liu, X.; Jiang, Y.; Chen, Y.X.; Chen, Y.F.; Lu, H.; Qian, J.; Wang, Z.; et al. Continuous tracking of COVID-19 patients’ immune status. Int. Immunopharmacol. 2020, 89, 107034.
- Alam, M.; Hasan, G.M.; Hassan, M.I. A review on the role of TANK-binding kinase 1 signaling in cancer. Int. J. Biol. Macromol. 2021, 183, 2364–2375.
- De Bock, M.; Thorstensen, E.B.; Derraik, J.G.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (O lea europaea L.) leaf extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085.
- Wang, Y.; Li, L.; Deng, S.; Liu, F.; He, Z. Ursolic acid ameliorates inflammation in cerebral ischemia and reperfusion injury possibly via high mobility group box 1/Toll-like receptor 4/NFκB pathway. Front. Neurol. 2018, 9, 253.
- Akash, M.S.H.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531.
- Fernandes, J.V.; Cobucci, R.N.O.; Jatobá, C.A.N.; de Medeiros Fernandes, T.A.A.; de Azevedo, J.W.V.; de Araújo, J.M.G. The role of the mediators of inflammation in cancer development. Pathol. Oncol. Res. 2015, 21, 527–534.
- Kim, J.Y.; Kim, Y.M.; Park, J.M.; Han, Y.M.; Lee, K.C.; Hahm, K.B.; Hong, K. Cancer preventive effect of recombinant TRAIL by ablation of oncogenic inflammation in colitis-associated cancer rather than anticancer effect. Oncotarget 2018, 9, 1705–1716.
- Jang, Y.; Kim, E.K.; Shim, W.S. Phytotherapeutic effects of the fruits of Poncirus trifoliata (L.) Raf. on cancer, inflammation, and digestive dysfunction. Phytother. Res. 2018, 32, 616–624.
- Lee, H.J.; Park, J.M.; Han, Y.M.; Gil, H.K.; Kim, J.; Chang, J.Y.; Jeong, M.; Go, E.-J.; Hahm, K.B. The role of chronic inflammation in the development of gastrointestinal cancers: Reviewing cancer prevention with natural anti-inflammatory intervention. Expert. Rev. Gastroenterol. Hepatol. 2016, 10, 129–139.
- Kim, E.Y.; Kim, N.; Kim, Y.S.; Seo, J.Y.; Park, I.; Ahn, H.K.; Jeong, Y.M.; Kim, J.H. Prognostic Significance of Modified Advanced Lung Cancer Inflammation Index (ALI) in Patients with Small Cell Lung Cancer_ Comparison with Original ALI. PLoS ONE 2016, 11, e0164056.
- Costa, J.F.O.; Barbosa-Filho, J.M.; de Azevedo Maia, G.L.; Guimarães, E.T.; Meira, C.S.; Ribeiro-dos-Santos, R.; de Carvalho, L.C.P.; Soares, M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014, 23, 469–474.
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. Immunopathology, host-virus genome interactions, and effective vaccine development in SARS-CoV-2. Comput. Struct. Biotechnol. J. 2020, 18, 3774–3787.
- Subedi, L.; Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771.
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid.-Based Complementary Altern. Med. 2015, 2015, 620472.
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194.
- Kumar, S.; Rana, R.; Yadav, D.K. Atomic-scale modeling of the effect of lipid peroxidation on the permeability of reactive species. J. Biomol. Struct. Dyn. 2021, 39, 1284–1294.
- Zerin, T.; Lee, M.; Jang, W.S.; Nam, K.W.; Song, H.Y. Anti-inflammatory potential of ursolic acid in Mycobacterium tuberculosis-sensitized and Concanavalin A-stimulated cells. Mol. Med. Rep. 2016, 13, 2736–2744.
- Huang, Q.; Chen, H.; Ren, Y.; Wang, Z.; Zeng, P.; Li, X.; Wang, J.; Zheng, X. Anti-hepatocellular carcinoma activity and mechanism of chemopreventive compounds: Ursolic acid derivatives. Pharm. Biol. 2016, 54, 3189–3196.
- Bowen-Forbes, C.S.; Mulabagal, V.; Liu, Y.; Nair, M.G. Ursolic acid analogues: Non-phenolic functional food components in Jamaican raspberry fruits. Food. Chem. 2009, 116, 633–637.
- Dwivedi, G.R.; Rai, R.; Pratap, R.; Singh, K.; Pati, S.; Sahu, S.N.; Kant, J.; Darokar, M.P.; Yadav, D.K. Drug resistance reversal potential of multifunctional thienopyran via potentiation of antibiotics in MDR P. aeruginosa. Biomed. Pharmacother. 2021, 142, 112084.
- Wei, Z.-Y.; Chi, K.-Q.; Wang, K.-S.; Wu, J.; Liu, L.-P.; Piao, H.-R. Design, synthesis, evaluation, and molecular docking of ursolic acid derivatives containing a nitrogen heterocycle as anti-inflammatory agents. Bioorganic Med. Chem. Lett. 2018, 28, 1797–1803.
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. Recent Advances in Pathophysiology, Drug Development and Future Perspectives of SARS-CoV-2. Front. Cell. Dev. Biol. 2020, 8, 580202.
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxidative Med. Cell. Longev. 2019, 2019, 8512048.
- Wang, Y.-J.; Lu, J.; Wu, D.-M.; Zheng, Z.-H.; Zheng, Y.-L.; Wang, X.-H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.-F. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165.
- Habtemariam, S. Iridoids and other monoterpenes in the Alzheimer’s brain: Recent development and future prospects. Molecules 2018, 23, 117.
- Na, H.; Mok, C.; Lee, J. Effects of plasma treatment on the oxidative stability of vegetable oil containing antioxidants. Food Chem. 2020, 302, 125306.
- Jeong, S.Y.; Gu, X.; Jeong, K.W. Photoactivation of N-retinylidene-N-retinylethanolamine compromises autophagy in retinal pigmented epithelial cells. Food Chem. Toxicol. 2019, 131, 110555.
- Islam, M.Z.; Park, B.J.; Lee, Y.T. Effect of salinity stress on bioactive compounds and antioxidant activity of wheat microgreen extract under organic cultivation conditions. Int. J. Biol. Macromol. 2019, 140, 631–636.
- Cho, H.T.; Kim, J.H.; Heo, W.; Lee, H.S.; Lee, J.J.; Park, T.S.; Lee, J.H.; Kim, Y.J. Explosively Puffed Ginseng Ameliorates Ionizing Radiation-Induced Injury of Colon by Decreasing Oxidative Stress-Related Apoptotic Cell Execution in Mice. J. Med. Food 2019, 22, 490–498.
- Habtemariam, S. Going back to the good old days: The merit of crude plant drug mixtures in the 21st century. Int. J. Complementary Altern. Med. 2017, 6, 1–5.
- Wahedi, H.M.; Chae, J.K.; Subedi, L.; Kang, M.C.; Cho, H.; Kim, S.; Kim, S.Y. NED416, a novel synthetic Sirt1 activator, promotes cutaneous wound healing via the MAPK/Rho pathway. Int. J. Mol. Med. 2020, 46, 149–158.
- Li, J.-S.; Wang, W.-J.; Sun, Y.; Zhang, Y.-H.; Zheng, L. Ursolic acid inhibits the development of nonalcoholic fatty liver disease by attenuating endoplasmic reticulum stress. Food Funct. 2015, 6, 1643–1651.
- Li, J.; Li, N.; Yan, S.; Liu, M.; Sun, B.; Lu, Y.; Shao, Y. Ursolic acid alleviates inflammation and against diabetes-induced nephropathy through TLR4-mediated inflammatory pathway. Mol. Med. Rep. 2018, 18, 4675–4681.
- Ullevig, S.L.; Zhao, Q.; Zamora, D.; Asmis, R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 2011, 219, 409–416.
- Xiang, M.; Wang, J.; Zhang, Y.; Ling, J.; Xu, X. Attenuation of aortic injury by ursolic acid through RAGE-Nox-NFκB pathway in streptozocin-induced diabetic rats. Arch. Pharmacal Res. 2012, 35, 877–886.
