The medical procedures in endodontics are time-consuming and mostly require several visits to be able to achieve the proper result. In this field of dentistry, there are still major issues about the removal of the mostly bacterial infection from the dental root canals. It has been confirmed that nanoparticles are much more efficient than traditional materials and appear to have superior properties when it comes to surface chemistry and bonding. Their unique antibacterial properties are also promising features in every medical procedure, especially in endodontics. High versatility of use of nanomaterials makes them a powerful tool in dental clinics, in a plethora of endodontic procedures, including pulp regeneration, drug delivery, root repair, disinfection, obturation and canal filling.
- nanomaterials
- endodontics
- dentistry
1. Introduction
One of the branches of dentistry that deals with the morphology and physiology of the endodontium is endodontics. It combines such aspects of this field as etiology, pathology, epidemiology, prophylaxis and, above all, treatment of endodontic and periapical diseases. Depending on the complexity of the case, the treatment process may be carried out at one or more visits. Due to the difficulty of maintaining the sterility of the operator’s work area, nanomaterials are increasingly used. Thanks to the expanding variety of nanoparticles, such as bioactive glass, zirconia, chitosan, hydroxyapatite, silver particles, zinc oxide, the properties of materials used in dentistry, such as durability, tissue regeneration and bactericidal properties, can be improved.
2. Classification of Nanomaterials, Materials Modification
2.1. Quantum Influence on Nanomaterials
2.1.1. Quantum Confinement Effects
2.1.2. Surface Effects
2.2. Chitosan Nanoparticles
2.3. Hydroxyapatite (HAp)
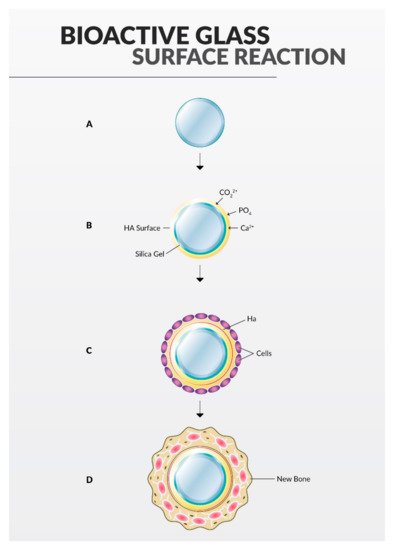
2.4. Bioactive Glass
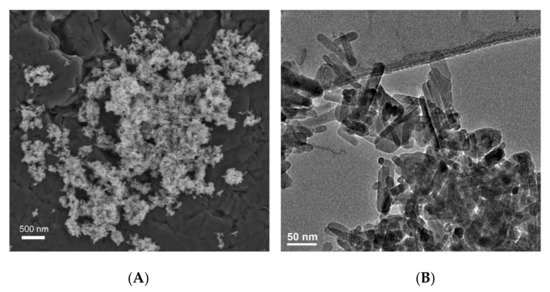
2.5. Zirconia (Zr)
2.6. Nanosilver
2.7. Zinc Oxide (ZnO)
2.8. Exosomes
Various MSCs participate in the process of pulp formation, such as dental pulp stem cells (DPSC), human exfoliated deciduous tooth stem cells (SHED), apical papilla stem cells (SCAP), and dental alveolar progenitor cells (DFPC) [49]. Zhou et al. [50] proved that microballoons from dental pulp stem cells (DPSCs) can be used in regenerative endodontic therapy due to their pro-angiogenic effect.
2.9. Graphene
2.10. Nanopolymers
3. Clinical Applications
3.1. Sealers
3.2. Obturating Materials
3.3. Nano-Size Related Drug Delivery Applications in Endodontics
3.4. Root Repair Materials
3.5. Nanoparticle-Based Disinfection in Endodontics
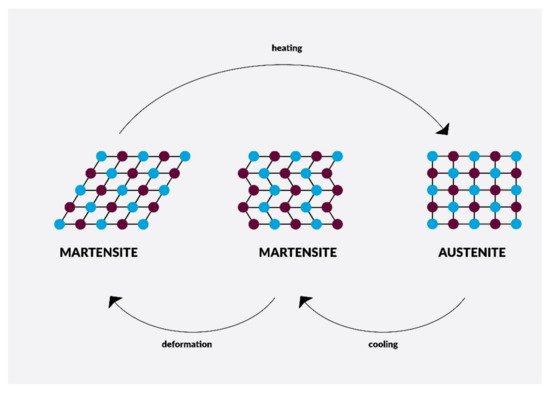
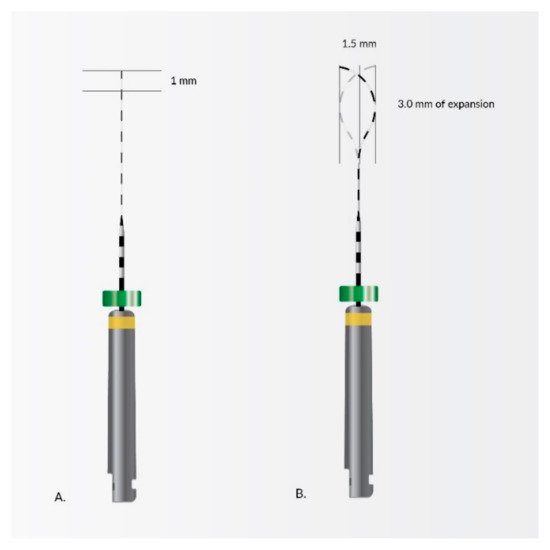
4. Nanomaterials in Endodontic Instruments and Their Effects
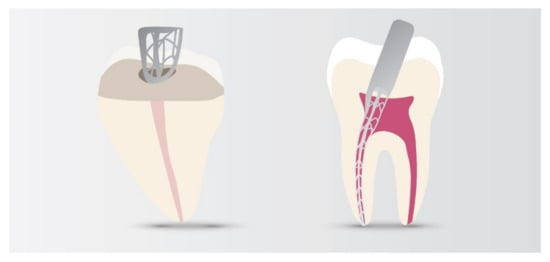
5. Nanoapplications for Repair and Pulp Regeneration
This entry is adapted from the peer-reviewed paper 10.3390/ma14185296
References
- Veerapandian, M.; Yun, K. Functionalization of biomolecules on nanoparticles: Specialized for antibacterial applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667.
- Jhajharia, K.; Mehta, L.; Parolia, A.; Shetty, K. Biofilm in endodontics: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 1.
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592.
- Neikov, O. Quantum Confinement Effect—An Overview. Available online: https://www.sciencedirect.com/topics/engineering/quantum-confinement-effect (accessed on 21 April 2021).
- Cassidy, J.; Zamkov, M. Nanoshell quantum dots: Quantum confinement beyond the exciton Bohr radius. J. Chem. Phys. 2020, 152, 110902.
- Barmparis, G.D.; Kopidakis, G.; Remediakis, I.N. Shape-dependent single-electron levels for Au nanoparticles. Materials 2016, 9, 301.
- Yu, X.; Zhan, Z. The effects of the size of nanocrystalline materials on their thermodynamic and mechanical properties. Nanoscale Res. Lett. 2014, 9, 1–6.
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556.
- Giebultowicz, T. Breathing life into an old model. Nature 2000, 408, 299–301.
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81.
- Callaway, J. Model for lattice thermal conductivity at low temperatures. Phys. Rev. 1959, 113, 1046–1051.
- Wang, Z.; Alaniz, J.E.; Jang, W.; Garay, J.E.; Dames, C. Thermal conductivity of nanocrystalline silicon: Importance of grain size and frequency-dependent mean free paths. Nano Lett. 2011, 11, 2206–2213.
- Rowe, D.M. (Ed.) Thermoelectrics Handbook: Macro to Nano, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006.
- Slack, G.A.; Galginaitis, S. Thermal conductivity and phonon scattering by magnetic impurities in CdTe. Phys. Rev. 1964, 133, A253.
- Zhang, L.; Huang, H. Young’s moduli of ZnO nanoplates: Ab initio determinations. Appl. Phys. Lett. 2006, 89, 183111.
- Nanda, K.K. Size-dependent density of nanoparticles and nanostructured materials. Phys. Lett. A 2012, 376, 3301–3302.
- Safaei, A. Shape, structural, and energetic effects on the cohesive energy and melting point of nanocrystals. J. Phys. Chem. C 2010, 114, 13482–13496.
- Jiang, Q.; Li, J.C.; Chi, B.Q. Size-dependent cohesive energy of nanocrystals. Chem. Phys. Lett. 2002, 366, 551–554.
- Sun, C.Q.; Wang, Y.; Tay, B.K.; Li, S.; Huang, H.; Zhang, Y.B. Correlation between the melting point of a nanosolid and the cohesive energy of a surface atom. J. Phys. Chem. B 2002, 106, 10701–10705.
- Guisbiers, G.; Buchaillot, L. Modeling the melting enthalpy of nanomaterials. J. Phys. Chem. C 2009, 113, 3566–3568.
- Guisbiers, G. Review on the analytical models describing melting at the nanoscale. J. Nanosci. Lett. 2012, 2, 8.
- Gelb, L.D.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Phase separation in confined systems. Rep. Prog. Phys. 2000, 63, 727.
- Berry, R.S. Phases and Phase Changes of Small Systems. In Theory of Atomic and Molecular Clusters; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1–26.
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28.
- Del Carpio-Perochena, A.; Kishen, A.; Shrestha, A.; Bramante, C.M. Antibacterial Properties Associated with Chitosan Nanoparticle Treatment on Root Dentin and 2 Types of Endodontic Sealers. J. Endod. 2015, 41, 1353–1358.
- Wang, N.; Ji, Y.; Zhu, Y.; Wu, X.; Mei, L.; Zhang, H.; Deng, J.; Wang, S. Antibacterial effect of chitosan and its derivative on Enterococcus faecalis associated with endodontic infection. Exp. Ther. Med. 2020, 19, 3805.
- Ballal, N.V.; Kundabala, M.; Bhat, K.S.; Acharya, S.; Ballal, M.; Kumar, R. Susceptibility of Candida albicans and Enterococcus faecalis to Chitosan, Chlorhexidine gluconate and their combination in vitro. Aust. Endod. J. 2009, 35, 29–33.
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084.
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449.
- Sebdani, M.M.; Fathi, M.H. Novel hydroxyapatite-forsterite-bioglass nanocomposite coatings with improved mechanical properties. J. Alloys Compd. 2011, 509, 2273–2276.
- Sung, Y.M.; Lee, J.C.; Yang, J.W. Crystallization and sintering characteristics of chemically precipitated hydroxyapatite nanopowder. J. Cryst. Growth 2004, 262, 467–472.
- Fathi, M.H.; Hanifi, A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol-gel method. Mater. Lett. 2007, 61, 3978–3983.
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997.
- Al-Hazmi, F.; Alnowaiser, F.; Alghamdi, A.A.; Aly, M.M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A new large—Scale synthesis of magnesium oxide nanowires: Structural and antibacterial properties. Superlattices Microstruct. 2012, 52, 200–209.
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials 2017, 10, 590.
- Piotrowski, G.; Hench, L.L.; Allen, W.C.; Miller, G.J. Mechanical studies of the bone bioglass interfacial bond. J. Biomed. Mater. Res. 1975, 9, 47–61.
- Deville, S.; Gremillard, L.; Chevalier, J.; Fantozzi, G. A critical comparison of methods for the determination of the aging sensitivity in biomedical grade yttria-stabilized zirconia. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 239–245.
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543.
- Kumar, G.; Shivrayan, A. Comparative study of mechanical properties of direct core build-up materials. Contemp. Clin. Dent. 2015, 6, 16–20.
- Asakawa, Y.; Takahashi, H.; Iwasaki, N.; Kobayashi, M. Effect of ultraviolet light irradiation period on bond strengths between fber-reinforced composite post and core build-up composite resin. Dent. Mater. J. 2014, 33, 133–140.
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 1–10.
- Chladek, G.; Barszczewska-Rybarek, I.; Lukaszczyk, J. Developing the procedure of modifying the denture soft liner by silver nanoparticles. Acta Bioeng. Biomech. 2012, 14, 23–29.
- Senges, C.; Wrbas, K.T.; Altenburger, M.; Follo, M.; Spitzmüller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252.
- George, S.; Basrani, B.; Kishen, A. Possibilities of gutta-percha-centered infection in endodontically treated teeth: An in vitro study. J. Endod. 2010, 36, 1241–1244.
- Siqueira, J.F.; Favieri, A.; Gahyva, S.M.M.; Moraes, S.R.; Lima, K.C.; Lopes, H.P. Antimicrobial activity and flow rate of newer and established root canal sealers. J. Endod. 2000, 26, 274–277.
- Ørstavik, D. Antibacterial properties of root canal sealers, cements and pastes. Int. Endod. J. 1981, 14, 125–133.
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An Investigation on the Antibacterial and Antibiofilm Efficacy of Cationic Nanoparticulates for Root Canal Disinfection. J. Endod. 2008, 34, 1515–1520.
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182.
- Shah, N.; Logani, A.; Bhaskar, U.; Aggarwal, V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: A pilot clinical study. J. Endod. 2008, 34, 919–925.
- Zhou, H.; Li, X.; Yin, Y.; He, X.-T.; An, Y.; Tian, B.-M.; Hong, Y.-L.; Wu, L.-A.; Chen, F.-M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 1–18.
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial Activity against Staphylococcus Aureus of Titanium Surfaces Coated with Graphene Nanoplatelets to Prevent Peri-Implant Diseases. An In-Vitro Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 1568.
- Beyth, N.; Houri-Haddad, Y.; Baraness-Hadar, L.; Yudovin-Farber, I.; Domb, A.J.; Weiss, E.I. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 2008, 29, 4157–4163.
- Abramovitz, I.; Wisblech, D.; Zaltsman, N.; Weiss, E.I.; Beyth, N. Intratubular Antibacterial Effect of Polyethyleneimine Nanoparticles: An Ex Vivo Study in Human Teeth. J. Nanomater. 2015, 2015.
- Siqueira, J.F.; Guimarães-Pinto, T.; Rôças, I.N. Effects of Chemomechanical Preparation with 2.5% Sodium Hypochlorite and Intracanal Medication with Calcium Hydroxide on Cultivable Bacteria in Infected Root Canals. J. Endod. 2007, 33, 800–805.
- AlShwaimi, E.; Bogari, D.; Ajaj, R.; Al-Shahrani, S.; Almas, K.; Majeed, A. In Vitro Antimicrobial Effectiveness of Root Canal Sealers against Enterococcus faecalis: A Systematic Review. J. Endod. 2016, 42, 1588–1597.
- Kontakiotis, E.G.; Wu, M.-K.; Wesselink, P.R. Effect of sealer thickness on long-term sealing ability: A 2-year follow-up study. Int. Endod. J. 2003, 30, 307–312.
- Schäfer, E.; Bering, N.; Bürklein, S. Selected physicochemical properties of AH Plus, EndoREZ and RealSeal SE root canal sealers. Odontology 2015, 103, 61–65.
- Lodiene, G.; Morisbak, E.; Bruzell, E.; Ørstavik, D. Toxicity evaluation of root canal sealers in vitro. Int. Endod. J. 2008, 41, 72–77.
- Barros, J.; Silva, M.G.; Rodrigues, M.A.; Alves, F.R.F.; Lopes, M.A.; Pina-Vaz, I.; Siqueira, J.F. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int. Endod. J. 2014, 47, 725–734.
- Abramovitz, I.; Beyth, N.; Weinberg, G.; Borenstein, A.; Polak, D.; Kesler-Shvero, D.; Houri-Haddad, Y. In vitro biocompatibility of endodontic sealers incorporating antibacterial nanoparticles. J. Nanomater. 2012, 2012.
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426.
- Tomson, R.M.E.; Polycarpou, N.; Tomson, P.L. Contemporary obturation of the root canal system. Br. Dent. J. 2014, 216, 315–322.
- Whitworth, J. Methods of filling root canals: Principles and practices. Endod. Top. 2005, 12, 2–24.
- Vishwanath, V.; Rao, H. Gutta-percha in—A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216.
- Lee, D.K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.Y.; Shi, W.; Hong, C.; Tetradis, S.; Kim, Y.; et al. Nanodiamond-Gutta Percha Composite Biomaterials for Root Canal Therapy. ACS Nano 2015, 9, 11490–11501.
- Melker, K.B.; Vertucci, F.J.; Rojas, M.F.; Progulske-Fox, A.; Bélanger, M. Antimicrobial efficacy of medicated root canal filling materials. J. Endod. 2006, 32, 148–151.
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; Da Cruz, A.D.; Poiate, E.; Poiate, I.A.V.P. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015.
- Shantiaee, Y.; Dianat, O.; Mohammadkhani, H.; Akbarzadeh Baghban, A. Cytotoxicity Comparison of Nanosilver Coated Gutta-Percha with Guttaflow and Normal Gutta-Percha on L929 Fibroblast with Mtt Assay. J. Dent. Sch. Shahid Beheshti Univ. Med. Sci. 2011, 29, 63–69.
- Wang, T.; Zhang, D.; Sun, D.; Gu, J. Current status of in vivo bioanalysis of nano drug delivery systems. J. Pharm. Anal. 2020, 10, 221–232.
- Renugalakshmi, A.; Sekar Vinothkumar, T.; Kandaswamy, D. Nanodrug Delivery Systems in Dentistry: A Review on Current Status and Future Perspectives. Curr. Drug Deliv. 2011, 8, 586–594.
- Pan, S.; Yu, H.; Yang, X.; Yang, X.; Wang, Y.; Liu, Q.; Jin, L.; Yang, Y. Application of Nanomaterials in Stem Cell Regenerative Medicine of Orthopedic Surgery. J. Nanomater. 2017, 2017.
- Damas, B.A.; Wheater, M.A.; Bringas, J.S.; Hoen, M.M. Cytotoxicity comparison of mineral trioxide aggregates and endosequence bioceramic root repair materials. J. Endod. 2011, 37, 372–375.
- Brzęcka, D.M.; Staniowski, T. Nowe materiały bioceramiczne do naprawy korzenia—Przegląd piśmiennictwa. Dent. Med. Probl. 2016, 53, 551–558.
- Alenazy, M.S.; Mosadomi, H.A.; Al-Nazhan, S.; Rayyan, M.R. Clinical considerations of nanobiomaterials in endodontics: A systematic review. Saudi Endod. J. 2018, 8, 163–169.
- Subramani, K.; Ahmed, W. (Eds.) Nanobiomaterials in Clinical Dentistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019.
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346.
- Pagonis, T.C.; Chen, J.; Fontana, C.R.; Devalapally, H.; Ruggiero, K.; Song, X.; Foschi, F.; Dunham, J.; Skobe, Z.; Yamazaki, H.; et al. Nanoparticle-based Endodontic Antimicrobial Photodynamic Therapy. J. Endod. 2010, 36, 322–328.
- Shaw, J.A.; Churchill, C.B.; Iadicola, M.A. Tips and Tricks for Characterizing Shape Memory Alloy Wire: Part 1-Differential Scanning Calorimetry and Basic Phenomena. Exp. Tech. 2008, 32, 55–62.
- Metzger, Z. The self- A djusting file (SAF) system: An evidence-based update. J. Conserv. Dent. 2014, 17, 401–419.
- Adini, A.R.; Feldman, Y.; Cohen, S.R.; Rapoport, L.; Moshkovich, A.; Redlich, M.; Moshonov, J.; Shay, B.; Tenne, R. Alleviating fatigue and failure of NiTi endodontic files by a coating containing inorganic fullerene-like WS2 nanoparticles. J. Mater. Res. 2011, 26, 1234–1242.
- Ahn, S.; Ha, J.H.; Kwak, S.W.; Kim, H.C. Advancement of mechanical properties of nickel-titanium rotary endodontic instruments by spring machining on the file shaft. Materials 2020, 13, 5246.
- Fioretti, F.; Mendoza-Palomares, C.; Avoaka-Boni, M.C.; Ramaroson, J.; Bahi, S.; Richert, L.; Granier, F.; Benkirane-Jessel, N.; Haikel, Y. Nano-odontology: Nanostructured assemblies for endodontic regeneration. J. Biomed. Nanotechnol. 2011, 7, 471–475.
- Schultz, P. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesis. Biomaterials 2005, 26, 2621–2630.
- Smith, I.O.; Liu, X.H.; Smith, L.A.; Ma, P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 226–236.
- Yang, X.; Yang, F.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J. Biomed. Mater. Res. Part A 2010, 93, 247–257.
- Wang, J.; Liu, X.; Jin, X.; Ma, H.; Hu, J.; Ni, L.; Ma, P.X. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(l-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010, 6, 3856–3863.
- Gupte, M.J.; Ma, P.X. Nanofibrous scaffolds for dental and craniofacial applications. J. Dent. Res. 2012, 91, 227–234.
