C. auris has unprecedently emerged as a multi-drug resistant fungal pathogen considered a serious global threat due to its potential to cause nosocomial outbreaks and deep-seated infections with staggering transmissibility and mortality, that has put in check the health authorities and institutions worldwide for more than a decade now. Due to its unique features not observed in other yeasts, it has been categorized as an urgent threat by the Centers for Disease Control and Prevention and the rest of international agencies. Moreover, epidemiological alerts have been released in view of the increase of healthcare-associated C. auris outbreaks in the context of the COVID-19 pandemic. This review summarizes the current evidence on C. auris since its first description, from virulence to treatment and outbreak control, and highlights the knowledge gaps and future directions for research efforts.
- Candida auris
- candidaemia
- virulence
- pathogenesis
- Resistance
- Treatment
1. Introduction
Candida auris is an emergent species which, as a consequence of its multidrug resistance to common antifungals [1][2][3], difficult identification with conventional biochemical microbiological techniques [4][5], high transmissibility, surface survival [6], and environmental adaptability [7][8], has been associated with serious nosocomial IFI with high mortality and is extremely difficult control in many countries [2][7][9][10][11].
2. Importance and Chronology of C. auris Emergence
3. Hypotheses on the Origin of C. auris
4. Microbiological Features of C. auris
4.1. Phylogeny
4.2. Culture, Growth, and Phenotypes
-
Non-aggregative phenotype: yeast cells arrange as isolated or, sometimes, coupled cells, similarly to other Candida species.
-
Aggregative phenotype: some isolates keep daughter cells attached after budding, creating large aggregates that cannot be separated by physical disruption after vigorous vortexing for several minutes.
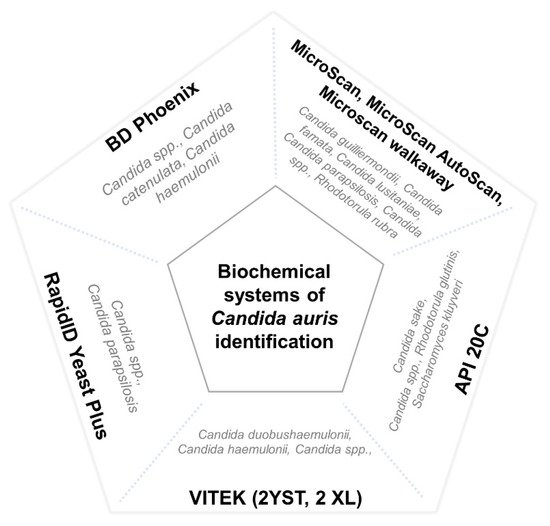
4.3. Difficulties in C. auris Identification
4.4. Virulence
| Organism | Virulence Results | Reference |
|---|---|---|
| C. elegans | C. hameulonii < C. auris = C. albicans | [90] |
| C. elegans | Non-Ag C. auris > Ag-C. auris | [58] |
| D. rerio | C. auris > C. albicans > C. haemulonii | [88] |
| D. melanogaster | C. auris > C. albicans Non-Ag C. auris = Ag C. auris > C. albicans |
[91] |
| G. mellonella | C. albicans > C. auris > C. parapsilosis Non-Ag C. auris > Ag-C. auris Non-invasive isolates = invasive isolates |
[52] |
| G. mellonella | C. auris ≥ C. albicans Non-Ag C. auris > Ag-C. auris |
[34] |
| G. mellonella | Non-Ag C. auris ≥ C. albicans and C. glabrata Ag-C. auris = C. glabrata |
[53] |
| G. mellonella | C. auris < C. albicans Non-ag C. auris = Ag C. auris |
[57] |
| G. mellonella | C. auris < C. albicans Non-ag C. auris = Ag C. auris |
[54] |
| G. mellonella | C. albicans > C. auris > C. haemulonii | [55] |
| G. mellonella | Non-Ag C. auris > Ag-C. auris Blood isolates > respiratory and urine isolates |
[58] |
| Neutropenic Mus musculus | C. auris = C. haemulonii | [55] |
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9102177
References
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921.
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290.
- Ku, T.S.N.; Walraven, C.J.; Lee, S.A. Candida auris: Disinfectants and Implications for Infection Control. Front. Microbiol. 2018, 9, 726.
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: Host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70, 1318.
- Iguchi, S.; Itakura, Y.; Yoshida, A.; Kamada, K.; Mizushima, R.; Arai, Y.; Uzawa, Y.; Kikuchi, K. Candida auris: A pathogen difficult to identify, treat, and eradicate and its characteristics in Japanese strains. J. Infect. Chemother. 2019, 25, 743–749.
- Welsh, R.M.; Bentz, M.L.; Shams, A.; Houston, H.; Lyons, A.; Rose, L.J.; Litvintseva, A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005.
- Eyre, D.W.; Sheppard, A.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331.
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Morel, H.M.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505.
- Ruiz-Gaitán, A.; Martínez, H.; Moret, A.M.; Calabuig, E.; Tasias, M.; Alastruey-Izquierdo, A.; Zaragoza, O.; Mollar, J.; Frasquet, J.; Salavert-Lletí, M.; et al. Detection and treatment of Candida auris in an outbreak situation: Risk factors for developing colonization and candidemia by this new species in critically ill patients. Expert Rev. Anti-Infect. Ther. 2019, 17, 295–305.
- Lockhart, S.R.; Berkow, E.L.; Chow, N.; Welsh, R.M. Candida auris for the Clinical Microbiology Laboratory: Not Your Grandfather’s Candida Species. Clin. Microbiol. Newsl. 2017, 39, 99–103.
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 1–7.
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44, Erratum in Microbiol. Immunol. 2018, 62, 205.
- European Center for Diseases Control (ECDC). Candida auris in Healthcare Settings—Europe (2016). Available online: http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_Disp (accessed on 14 October 2021).
- Public Health of England. Research and Analysis: Candida auris Identified in England. 2016. Available online: https://www.gov.uk/government/publications/candida-auris-emergence-in-england/candida-auris-identified-in-england (accessed on 14 October 2021).
- Pan American Health Organization/World Health Organization. PAHO/WHO. Epidemiological Alerts and Reports: C. auris Outbreaks in Health Care Services. 2016. Available online: https://www.paho.org/hq/dmdocuments/2016/2016-oct-3-phe-candida-auris-epi-alert.pdf (accessed on 4 September 2021).
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. Centers for Disease Control. December 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 14 October 2021).
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; De Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. Morb. Mortal. Weekly Rep. 2021, 70, 56–57.
- Hanson, B.M.; Dinh, A.Q.; Tran, T.T.; Arenas, S.; Pronty, D.; Gershengorn, H.B.; Ferreira, T.; Arias, C.A.; Shukla, B.S. Candida auris Invasive Infections during a COVID-19 Case Surge. Antimicrob. Agents Chemother. 2021, 65, AAC0114621.
- Magnasco, L.; Mikulska, M.; Giacobbe, D.; Taramasso, L.; Vena, A.; Dentone, C.; Dettori, S.; Tutino, S.; Labate, L.; Di Pilato, V.; et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris During the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms 2021, 9, 95.
- Rodriguez, J.Y.; Le Pape, P.; Lopez, O.; Esquea, K.; Labiosa, A.L.; Alvarez-Moreno, C. Candida auris: A latent threat to critically ill patients with COVID-19. Clin. Infect. Dis. 2020, 18, ciaa1595.
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696.
- Villanueva-Lozano, H.; Treviño-Rangel, R.D.J.; González, G.M.; Ramírez-Elizondo, M.T.; Lara-Medrano, R.; Aleman-Bocanegra, M.C.; Guajardo-Lara, C.E.; Gaona-Chávez, N.; Castilleja-Leal, F.; Torre-Amione, G.; et al. Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin. Microbiol. Infect. 2021, 27, 813–816.
- Allaw, F.; Zahreddine, N.K.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.; Dbaibo, G.; Kanj, S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157.
- de Almeida, J.; Francisco, E.; Hagen, F.; Brandão, I.; Pereira, F.; Dias, P.P.; Costa, M.D.M.; Jordão, R.D.S.; de Groot, T.; Colombo, A. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220.
- Pemán, J.; Ruiz-Gaitán, A.; García-Vidal, C.; Salavert, M.; Ramírez, P.; Puchades, F.; García-Hita, M.; Alastruey-Izquierdo, A.; Quindós, G. Fungal co-infection in COVID-19 patients: Should we be concerned? Rev. Iberoam. Micol. 2020, 37, 41–46.
- Chowdhary, A.; Sharma, A. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 175–176.
- Lee, W.G.; Shin, J.H.; Uh, Y.; Kang, M.G.; Kim, S.H.; Park, K.H.; Jang, H.-C. First Three Reported Cases of Nosocomial Fungemia Caused by Candida auris. J. Clin. Microbiol. 2011, 49, 3139–3142.
- Sharma, M.; Chakrabarti, A. On the Origin of Candida auris: Ancestor, Environmental Stresses, and Antiseptics. mBio 2020, 11, e02102-20.
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19.
- Chow, N.A.; De Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 1780–1781.
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2018, 57, 1–12, Erratum in Med. Mycol. 2019, 57, e7.
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19.
- Lamoth, F.; Kontoyiannis, D.P. The Candida auris Alert: Facts and Perspectives. J. Infect. Dis. 2017, 217, 516–520.
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16.
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. Environmental Candida auris and the Global Warming Emergence Hypothesis. mBio 2021, 12, e00360-21.
- Misseri, G.; Ippolito, M.; Cortegiani, A. Global warming “heating up” the ICU through Candida auris infections: The climate changes theory. Crit Care 2019, 23, 416.
- Casadevall, A. Fungi and the Rise of Mammals. PLoS Pathog. 2012, 8, e1002808.
- Robert, V.A.; Casadevall, A. Vertebrate Endothermy Restricts Most Fungi as Potential Pathogens. J. Infect. Dis. 2009, 200, 1623–1626.
- Arora, P.; Singh, P.; Wang, Y.; Yadav, A.; Pawar, K.; Singh, A.; Padmavati, G.; Xu, J.; Chowdhary, A. Environmental Isolation of Candida auris from the Coastal Wetlands of Andaman Islands, India. mBio 2021, 12, e03181-20.
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 1–13.
- Chybowska, A.D.; Childers, D.; Farrer, R.A. Nine Things Genomics Can Tell Us About Candida auris. Front. Genet. 2020, 11, 351.
- Cendejas-Bueno, E.; Kolecka, A.; Alastruey-Izquierdo, A.; Theelen, B.; Groenewald, M.; Kostrzewa, M.; Cuenca-Estrella, M.; Gómez-López, A.; Boekhout, T. Reclassification of the Candida haemulonii Complex as Candida haemulonii (C. haemulonii Group I), C. duobushaemulonii sp. nov. (C. haemulonii Group II), and C. haemulonii var. vulnera var. nov.: Three Multiresistant Human Pathogenic Yeasts. J. Clin. Microbiol. 2012, 50, 3641–3651.
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830.
- Muñoz, J.F.; Welsh, R.M.; Shea, T.; Batra, D.; Gade, L.; Howard, D.; Rowe, L.A.; Meis, J.F.; Litvintseva, A.P.; Cuomo, C.A. Clade-specific chromosomal rearrangements and loss of subtelomeric adhesins in Candida auris. Genetics 2021, 218, iyab029.
- Kim, M.; Shin, J.H.; Sung, H.; Lee, K.; Kim, E.; Ryoo, N.; Lee, J.; Jung, S.; Park, K.H.; Kee, S.J.; et al. Candida haemuloniiand Closely Related Species at 5 University Hospitals in Korea: Identification, Antifungal Susceptibility, and Clinical Features. Clin. Infect. Dis. 2009, 48, e57–e61.
- Rhodes, J.; Abdolrasouli, A.; Farrer, R.A.; Cuomo, C.A.; Aanensen, D.M.; Armstrong-James, D.; Fisher, M.C.; Schelenz, S. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg. Microbes Infect. 2018, 7, 1–12, Erratum in Emerg. Microbes. Infect. 2018, 7, 104.
- Kumar, A.; Sachu, A.; Mohan, K.; Vinod, V.; Dinesh, K.; Karim, S. Simple low cost differentiation of Candida auris from Candida haemulonii complex using CHROMagar Candida medium supplemented with Pal’s medium. Rev. Iberoam. Micol. 2017, 34, 109–111.
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genom. 2015, 16, 1–16.
- Chowdhary, A.; Kumar, V.A.; Sharma, C.; Prakash, A.; Agarwal, K.; Babu, R.; Dinesh, K.R.; Karim, S.; Singh, S.K.; Hagen, F.; et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 33, 919–926.
- Piedrahita, C.T.; Cadnum, J.L.; Jencson, A.L.; Shaikh, A.A.; Ghannoum, M.A.; Donskey, C.J. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and OtherCandidaSpecies. Infect. Control. Hosp. Epidemiol. 2017, 38, 1107–1109.
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637.
- Garcia-Bustos, V.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.; Sigona-Giangreco, I.A.; Cabañero-Navalon, M.D.; Sabalza-Baztán, O.; Salavert-Lletí, M.; Tormo, M.Á.; Pemán, J. Characterization of the Differential Pathogenicity of Candida auris in a Galleria mellonella Infection Model. Microbiol. Spectr. 2021, 3, e0001321.
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331.
- Romera, D.; Aguilera-Correa, J.-J.; García-Coca, M.; Mahillo-Fernández, I.; Viñuela-Sandoval, L.; García-Rodríguez, J.; Esteban, J. The Galleria mellonella infection model as a system to investigate the virulence of Candida auris strains. Pathog. Dis. 2020, 78, ftaa067.
- Muñoz, J.; Ramirez, L.; Dias, L.; Rivas, L.; Ramos, L.; Santos, A.; Taborda, C.; Parra-Giraldo, C. Pathogenicity Levels of Colombian Strains of Candida auris and Brazilian Strains of Candida haemulonii Species Complex in Both Murine and Galleria mellonella Experimental Models. J. Fungi 2020, 6, 104.
- Forgács, L.; Borman, A.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169.
- Carvajal, S.; Alvarado, M.; Rodríguez, Y.; Parra-Giraldo, C.; Varón, C.; Morales-López, S.; Rodríguez, J.; Gómez, B.; Escandón, P. Pathogenicity Assessment of Colombian Strains of Candida auris in the Galleria mellonella Invertebrate Model. J. Fungi 2021, 7, 401.
- Hernando-Ortiz, A.; Mateo, E.; Perez-Rodriguez, A.; de Groot, P.W.; Quindós, G.; Eraso, E. Virulence of Candida auris from different clinical origins in Caenorhabditis elegans and Galleria mellonella host models. Virulence 2021, 12, 1063–1075.
- Alfouzan, W.; Dhar, R.; Albarrag, A.; Al-Abdely, H. The emerging pathogen Candida auris: A focus on the Middle-Eastern countries. J. Infect. Public Health 2019, 12, 451–459.
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16.
- Hirayama, T.; Miyazaki, T.; Ito, Y.; Wakayama, M.; Shibuya, K.; Yamashita, K.; Takazono, T.; Saijo, T.; Shimamura, S.; Yamamoto, K.; et al. Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci. Rep. 2020, 10, 1–10.
- Borman, A.M. Of mice and men and larvae: Galleria mellonella to model the early host-pathogen interactions after fungal infection. Virulence 2017, 9, 9–12.
- Marcos-Zambrano, L.; Bordallo-Cardona, M.; Borghi, E.; Falleni, M.; Tosi, D.; Muñoz, P.; Escribano, P.; Guinea, J. Candida isolates causing candidemia show different degrees of virulence in Galleria mellonella. Med. Mycol. 2019, 58, 83–92.
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New Clonal Strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673.
- Azar, M.M.; Turbett, S.E.; Fishman, J.A.; Pierce, V.M. Donor-Derived Transmission of Candida auris During Lung Transplantation. Clin. Infect. Dis. 2017, 65, 1040–1042.
- Yue, H.; Bing, J.; Zheng, Q.; Zhang, Y.; Hu, T.; Du, H.; Wang, H.; Huang, G. Filamentation in Candida auris, an emerging fungal pathogen of humans: Passage through the mammalian body induces a heritable phenotypic switch. Emerg. Microbes Infect. 2018, 7, 1–13.
- Bravo Ruiz, G.; Ross, Z.K.; Gow, N.A.R.; Lorenz, A. Pseudohyphal Growth of the Emerging Pathogen Candida auris Is Triggered by Genotoxic Stress through the S Phase Checkpoint. mSphere 2020, 5, e00151-20.
- Fan, S.; Yue, H.; Zheng, Q.; Bing, J.; Tian, S.; Chen, J.; Ennis, C.L.; Nobile, C.J.; Huang, G.; Du, H. Filamentous growth is a general feature of Candida auris clinical isolates. Med. Mycol. 2021, 59, 734–740.
- Tian, S.; Rong, C.; Nian, H.; Li, F.; Chu, Y.; Cheng, S.; Shang, H. First cases and risk factors of super yeast Candida auris infection or colonization from Shenyang, China. Emerg. Microbes Infect. 2018, 7, 1–9.
- Wang, X.; Bing, J.; Zheng, Q.; Zhang, F.; Liu, J.; Yue, H.; Tao, L.; Du, H.; Wang, Y.; Wang, H.; et al. The first isolate of Candida auris in China: Clinical and biological aspects. Emerg. Microbes Infect. 2018, 7, 1–9.
- Sarma, S.; Kumar, N.; Govil, D.; Ali, T.; Mehta, Y.; Rattan, A. Candidemia caused by amphotericin B and Fluconazole resistant Candida auris. Indian J. Med. Microbiol. 2013, 31, 90–91.
- Khatamzas, E.; Madder, H.; Jeffery, K. Neurosurgical device-associated infections due to Candida auris - Three cases from a single tertiary center. J. Infect. 2019, 78, 409–421.
- Ruiz Gaitán, A.C.; Moret, A.; López Hontangas, J.L.; Molina, J.M.; Aleixandre López, A.I.; Cabezas, A.H.; Mollar Maseres, J.; Arcas, R.C.; Gómez Ruiz, M.D.; Chiveli, M.Á.; et al. Nosocomial fungemia by Candida auris: First four reported cases in continental Europe. Rev. Iberoam Micol. 2017, 34, 23–27.
- Heath, C.H.; Dyer, J.R.; Pang, S.; Coombs, G.W.; Gardam, D.J. Candida auris Sternal Osteomyelitis in a Man from Kenya Visiting Australia, 2015. Emerg. Infect. Dis. 2019, 25, 192–194.
- Alatoom, A.; Sartawi, M.; Lawlor, K.; AbdelWareth, L.; Thomsen, J.; Nusair, A.; Mirza, I. Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates. Int. J. Infect. Dis. 2018, 70, 36–37.
- Sharp, A.; Borman, A.; Perera, N.; Randle, M.; Braham, S.; Taori, S.; Charlett, A.; Guy, R.; Muller-Pebody, B.; Manuel, R.; et al. Assessing routine diagnostic methods for detecting Candida auris in England. J. Infect. 2018, 77, 448–454.
- Durante, A.J.; Maloney, M.H.; Leung, V.H.; Razeq, J.H.; Banach, D.B. Challenges in identifying Candida auris in hospital clinical laboratories: A need for hospital and public health laboratory collaboration in rapid identification of an emerging pathogen. Infect. Control. Hosp. Epidemiol. 2018, 39, 1015–1016.
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clin. Infect. Dis. 2016, 64, 141–143.
- Dewaele, K.; Lagrou, K.; Frans, J.; Hayette, M.-P.; Vernelen, K. Hospital Laboratory Survey for Identification of Candida auris in Belgium. J. Fungi 2019, 5, 84.
- Mahmoudi, S.; Afshari, S.A.K.; Gharehbolagh, S.A.; Mirhendi, H.; Makimura, K. Methods for identification of Candida auris, the yeast of global public health concern: A review. J. Mycol. Med. 2019, 29, 174–179.
- Sharma, C.; Kumar, N.; Meis, J.F.; Pandey, R.; Chowdhary, A. Draft Genome Sequence of a Fluconazole-Resistant Candida auris Strain from a Candidemia Patient in India. Genome Announc. 2015, 3, e00722-15.
- Alvarado, M.; Álvarez, J.B.; Lockhart, S.R.; Valentín, E.; Ruiz-Gaitán, A.C.; Eraso, E.; De Groot, P.W. Identification of Candida auris and related species by multiplex PCR based on unique GPI protein-encoding genes. Mycoses 2020, 64, 194–202.
- Martínez-Murcia, A.; Navarro, A.; Bru, G.; Chowdhary, A.; Hagen, F.; Meis, J.F. Internal validation of GPS™ MONODOSE CanAur dtec-qPCR kit following the UNE/EN ISO/IEC 17025:2005 for detection of the emerging yeast Candida auris. Mycoses 2018, 61, 877–884.
- Sattler, J.; Noster, J.; Brunke, A.; Plum, G.; Wiegel, P.; Kurzai, O.; Meis, J.; Hamprecht, A. Comparison of Two Commercially Available qPCR Kits for the Detection of Candida auris. J. Fungi 2021, 7, 154.
- de Jong, A.W.; Dieleman, C.; Carbia, M.; Tap, R.M.; Hagen, F. Performance of Two Novel Chromogenic Media for the Identification of Multidrug-Resistant Candida auris Compared with Other Commercially Available Formulations. J. Clin. Microbiol. 2021, 59, e03220-20.
- Sigona-Giangreco, I.A.; Garcia-Hita, M.; Ruiz-Gaitan, A.; Valentín-Gómez, E.; Garcia-Bustos, V.; Giner-Almaraz, M.; de Groot, P.; Peman, J. Usefulness of chromogenic media for presumptive identification of Candida auris. Material Intended for Publication; Unpublished work.
- Calderone, R.A.; Fonzi, W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001, 9, 327–335.
- Pharkjaksu, S.; Boonmee, N.; Mitrpant, C.; Ngamskulrungroj, P. Immunopathogenesis of Emerging Candida auris and Candida haemulonii Strains. J. Fungi 2021, 7, 725.
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89.
- Lima, S.L.; Rossato, L.; Melo, A.S.D.A. Evaluation of the potential virulence of Candida haemulonii species complex and Candida auris isolates in Caenorhabditis elegans as an in vivo model and correlation to their biofilm production capacity. Microb. Pathog. 2020, 148, 104461.
- Wurster, S.; Bandi, A.; Beyda, N.D.; Albert, N.D.; Raman, N.M.; Raad, I.I.; Kontoyiannis, D.P. Drosophila melanogaster as a model to study virulence and azole treatment of the emerging pathogen Candida auris. J. Antimicrob. Chemother. 2019, 74, 1904–1910.
- Brown, J.L.; Delaney, C.; Short, B.; Butcher, M.C.; McKloud, E.; Williams, C.; Kean, R.; Ramage, G. Candida auris Phenotypic Heterogeneity Determines Pathogenicity In Vitro. mSphere 2020, 5, e00371-20.
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229.
- Gago, S.; Garcia-Rodas, R.; Cuesta, I.; Mellado, E.; Alastruey-Izquierdo, A. Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosisvirulence in the non-conventional hostGalleria mellonella. Virulence 2013, 5, 278–285.
- Perdoni, F.; Falleni, M.; Tosi, D.; Cirasola, D.; Romagnoli, S.; Braidotti, P.; Clementi, E.; Bulfamante, G.; Borghi, E. A histological procedure to study fungal infection in the wax moth Galleria mellonella. Eur. J. Histochem. 2014, 58, 2428.
- Ames, L.; Duxbury, S.; Pawlowska, B.; Ho, H.-L.; Haynes, K.; Bates, S. Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy. Virulence 2017, 8, 1909–1917.
- Frenkel, M.; Mandelblat, M.; Alastruey-Izquierdo, A.; Mendlovic, S.; Semis, R.; Segal, E. Pathogenicity of Candida albicans isolates from bloodstream and mucosal candidiasis assessed in mice and Galleria mellonella. J. Mycol. Med. 2016, 26, 1–8.
- Mesa-Arango, A.C.; Forastiero, A.; Bernal-Martínez, L.; Cuenca-Estrella, M.; Mellado, E.; Zaragoza, O. The non-mammalian hostGalleria mellonellacan be used to study the virulence of the fungal pathogenCandida tropicalisand the efficacy of antifungal drugs during infection by this pathogenic yeast. Med. Mycol. 2013, 51, 461–472.
- Calvo, B.; Melo, A.S.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374.
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J. Clin. Microbiol. 2019, 57, e02055-18.
- Abdolrasouli, A.; Armstrong-James, D.; Ryan, L.; Schelenz, S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses 2017, 60, 758–763.
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses 2019, 62, 706–709.
- Bhattacharya, S.; Holowka, T.; Orner, E.P.; Fries, B.C. Gene Duplication Associated with Increased Fluconazole Tolerance in Candida auris cells of Advanced Generational Age. Sci. Rep. 2019, 9, 1–13.
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819.
- Staniszewska, M.; Monika, S. Virulence Factors in Candida species. Curr. Protein Pept. Sci. 2020, 21, 313–323.
- Jabeen, K.; Mal, P.B.; Tharwani, A.; Hashmi, M.; Farooqi, J. Persistence of Candida auris on latex and nitrile gloves with transmission to sterile urinary catheters‡. Med. Mycol. 2019, 58, 128–132.
- Castro, L.A.; Álvarez, M.I.; Giusiano, G.; Martínez, E. Candida auris infection in the central catheter of a patient without sepsis symptoms. Colomb. Medica 2019, 50, 293–298.
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere 2020, 5, e00910-19.
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and Profiling of Candida auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere 2018, 3, e00334-18.
- Kean, R.; Brown, J.; Gulmez, D.; Ware, A.; Ramage, G. Candida auris: A Decade of Understanding of an Enigmatic Pathogenic Yeast. J. Fungi 2020, 6, 30.
- Kean, R.; Ramage, G. Combined Antifungal Resistance and Biofilm Tolerance: The Global Threat of Candida auris. mSphere 2019, 4, e00458-19.
- Dominguez, E.G.; Zarnowski, R.; Choy, H.L.; Zhao, M.; Sanchez, H.; Nett, J.E.; Andes, D.R. Conserved Role for Biofilm Matrix Polysaccharides in Candida auris Drug Resistance. mSphere. 2019, 2, e00680-18.
- Kumar, D.; Banerjee, T.; Pratap, C.B.; Tilak, R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J. Infect. Dev. Ctries. 2015, 9, 435–437.
- Johnson, C.J.; Davis, J.M.; Huttenlocher, A.; Kernien, J.F.; Nett, J.E. Emerging Fungal Pathogen Candida auris Evades Neutrophil Attack. mBio 2018, 9, e01403-18.
