Selenium (Se) is a common trace metalloid found in the Earth’s crust. In 1817, chemist Jacob Berzelius isolated it for the first time, and since then, it has been known for its properties. Recent research has shown that treatment with Se at low concentrations has a beneficial influence on plant development and yield. Se may work as an essential factor by interfering with a several of physiological processes. It is a remarkable antioxidant and pro-oxidant agent of plants that helps to cope with a variety of abiotic stresses, such as salinity, drought, intense temperature fluctuations, toxic metals/metalloids, and other environmental pollutants and toxins.
- selenium
- nanoparticles
- plant nutrition
- agriculture
1. Selenium in Plants

1.1. Effects of Selenium in Plants
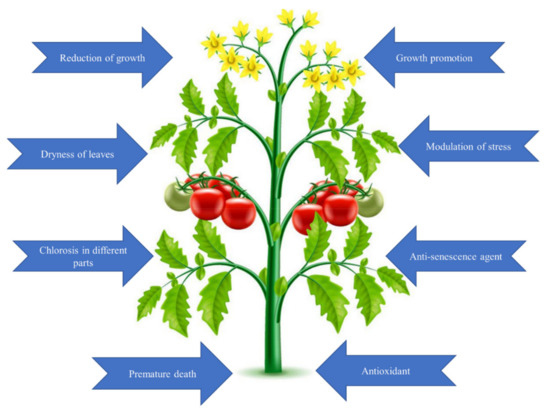
1.1.1. Beneficial Effects of Selenium in Plants
1.1.2. Harmful Effects of Selenium in Plants
2. Agricultural Use of Nano SeNPs
2.1. Fertilizer for Crops
2.2. Biofortification
2.3. Effect of SeNPs on Germination
This entry is adapted from the peer-reviewed paper 10.3390/agronomy11112229
References
- Wrobel, K.; Esperanza, M.G.; Barrientos, E.Y.; Escobosa, A.R.C. Different approaches in metabolomic analysis of plants exposed to selenium: A comprehensive review. Acta Physiol. Plant. 2020, 42, 125.
- Ralphs, M.H. Ecological relationships between poisonous plants and rangeland condition: A review. J. Range Manag. 2002, 55, 285–290.
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Rinklebe, J.; Tsang, D.C.W.; Tack, F.M.G.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566.
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2021.
- Mora, M.L.; Duran, P.; Acuna, A.J.; Cartes, P.; Demanet, R.; Gianfreda, L. Improving selenium status in plant nutrition and quality. J. Soil Sci. Plant Nutr. 2015, 15, 486–503.
- Brodowska, M.S.; Kurzyna-Szklarek, M.; Haliniarz, M. Selenium in the Environment. J. Elem. 2016, 21, 1173–1185.
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective Role of Selenium on Pepper Exposed to Cadmium Stress During Reproductive Stage. Biol. Trace Elem. Res. 2014, 160, 97–107.
- Hasanuzzaman, M.; Bhuyan, M.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Nahar, K.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170.
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290.
- Subramanyam, K.; Du Laing, G.; Van Damme, E.J.M. Sodium Selenate Treatment Using a Combination of Seed Priming and Foliar Spray Alleviates Salinity Stress in Rice. Front. Plant Sci. 2019, 10, 116.
- Jozwiak, W.; Politycka, B. Effect of Selenium on Alleviating Oxidative Stress Caused by a Water Deficit in Cucumber Roots. Plants 2019, 8, 217.
- Agbolade, J.O.; David, O.; Ajiboye, A.; Kioko, J.; Jolayemi, O.; Olawuni, I.; Ojo, M.; Akomolafe, G.; Adekoya, M.; Komolafe, R. Morpho-physiological effect of selenium on salinity-stressed wheat (Triticum aestivum L.). J. Biol. Res. 2019, 92, 7650.
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681.
- Ferreira, R.L.D.; Prado, R.D.; de Souza, J.P.; Gratao, P.L.; Tezotto, T.; Cruz, F.J.R. Oxidative Stress, Nutritional Disorders, and Gas Exchange in Lettuce Plants Subjected to Two Selenium Sources. J. Soil Sci. Plant Nutr. 2020, 20, 1215–1228.
- Pilon-Smits, E.A.H. On the Ecology of Selenium Accumulation in Plants. Plants 2019, 8, 197.
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624.
- Molnar, A.; Kolbert, Z.; Keri, K.; Feigl, G.; Ordog, A.; Szollosi, R.; Erdei, L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018, 148, 664–674.
- Naseem, M.; Anwar-ul-Haq, M.; Wang, X.K.; Farooq, N.; Awais, M.; Sattar, H.; Malik, H.A.; Mustafa, A.; Ahmad, J.; El-Esawi, M.A. Influence of Selenium on Growth, Physiology, and Antioxidant Responses in Maize Varies in a Dose-Dependent Manner. J. Food Qual. 2021, 2021, 6642018.
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 28.
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774.
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of Silicon and Silicon-Based Nanoparticles on Rhizosphere Microbiome, Plant Stress and Growth. Biology 2021, 10, 791.
- Jain, R.; Seder-Colomina, M.; Jordan, N.; Dessi, P.; Cosmidis, J.; van Hullebusch, E.D.; Weiss, S.; Farges, F.; Lens, P.N.L. Entrapped elemental selenium nanoparticles affect physicochemical properties of selenium fed activated sludge. J. Hazard. Mater. 2015, 295, 193–200.
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agriculture—A review. Sci. Total Environ. 2020, 712, 136365.
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147.
- Garza-Garcia, J.J.O.; Hernandez-Diaz, J.A.; Zamudio-Ojeda, A.; Leon-Morales, J.M.; Guerrero-Guzman, A.; Sanchez-Chipres, D.R.; Lopez-Velazquez, J.C.; Garcia-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace Elem. Res. 2021.
- Golubkina, N.A.; Folmanis, G.E.; Tananaev, I.G.; Krivenkov, L.V.; Kosheleva, O.V.; Soldatenko, A.V. Comparative Evaluation of Spinach Biofortification with Selenium Nanoparticles and Ionic Forms of the Element. Nanotechnol. Russ. 2017, 12, 569–576.
- Li, Y.X.; Zhu, N.L.; Liang, X.J.; Zheng, L.R.; Zhang, C.X.; Li, Y.F.; Zhang, Z.Y.; Gao, Y.X.; Zhao, J.T. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol. Environ. Saf. 2020, 189, 109955.
- Golubkina, N.A.; Folmanis, G.E.; Tananaev, I.G. Comparative evaluation of selenium accumulation by allium species after foliar application of selenium nanoparticles, sodium selenite and sodium selenate. Dokl. Biol. Sci. 2012, 444, 176–179.
- Wang, K.; Wang, Y.Q.; Li, K.; Wan, Y.N.; Wang, Q.; Zhuang, Z.; Guo, Y.B.; Li, H.F. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnology 2020, 18, 103.
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordeum Vulgare Barley Seeds. Coatings 2021, 11, 862.
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280.
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.U.R.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; Akram, A. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714.
- Bideshki, A.; Arvin, M.J.; Aien, A.; Hasandokht, M.R.; Khalighi, A. Interactive effects of Foliar 24-Epibrassinolide and selenium applications on yield, reduce nitrate accumulation and selenium enrichment in potato tuber in field. Cogent Food Agric. 2019, 5, 1690315.
