Visual plasticity is classically considered to occur essentially in the primary and secondary cortical areas. Subcortical visual areas such as the dorsal lateral geniculate nucleus (dLGN) or the superior colliculus (SC) have long been held as basic structures responsible for a stable and defined function. In this model, the dLGN was considered as a relay of visual information travelling from the retina to cortical areas and the SC as a sensory integrator orienting body movements towards visual targets. However, recent findings suggest that both dLGN and SC neurons express functional plasticity, adding unexplored layers of complexity to their previously attributed functions. The existence of neuronal plasticity at the level of visual subcortical areas redefines our approach of the visual system.
1. Introduction
1.1. Lateral Geniculate Nucleus and Superior Colliculus
In the mammalian visual system, the dorsal lateral geniculate nucleus (dLGN), a primary recipient structure of retinal inputs at the thalamic level, and the superior colliculus (SC), a lamellar structure involved in the comparison of multi-modal sensory information, constitute the main subcortical visual areas and occupy complementary functions. While the dLGN is involved in precise and conscious vision [
1,
2,
3], the SC is responsible for the initiation of eye and head movements towards specific objects [
4,
5,
6,
7]. Both structures receive direct inputs from retinal ganglion cells and from the primary visual cortex and communicate with each other (
Figure 1A,B). The dLGN is a thalamic nucleus whose organization varies across species. In primates and cats, the dLGN is organized in alternate monocular layers, whereas in rodents, no such clear lamination is visible. Rather, discrete monocular regions are identified with a large contralateral region surrounding a smaller ipsilateral projection zone (
Figure 1C). Two major functional types of dLGN relay neurons are found in primates, cats and rodents: cells that display linear summation (X-type or parvocellular neurons) and cells that display non-linear summation (Y-type or magnocellular neurons) [
8,
9,
10]. The linear type represents the overwhelming majority of dLGN neurons in primates, cats and rodents.
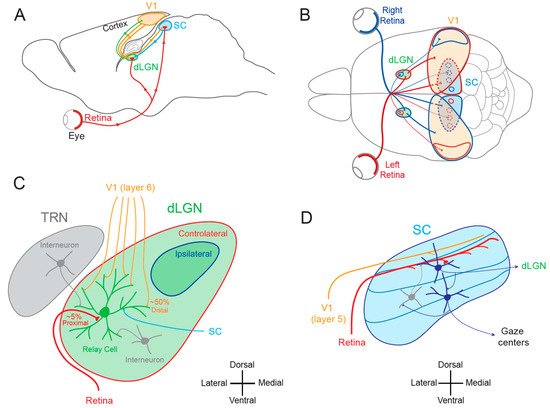
Figure 1. Visual pathways. (
A) Sagittal view of the rodent visual system. V1, primary visual area; SC, superior colliculus; dLGN, dorsal lateral geniculate nucleus. (
B) Superior view of the rodent visual system. Red, visual inputs from the left eye. Blue, visual inputs from the right eye. (
C) Simplified synaptic organization of visual inputs to rodent dLGN. The relay cell receives 3 types of excitatory inputs: (1) small amount (~5%) of functionally powerful contralateral inputs from the retina on proximal dendrite (red), (2) numerous (~50%) but functionally weak feed-back inputs from V1 (orange) on distal dendrites and (3) from the SC (light blue) on medial and distal dendrites. In addition, it is inhibited by interneurons located in the TRN (thalamic reticular nucleus) and in the dLGN (grey). (
D) Principal inputs and outputs of rodent SC neurons. In the superficial layer, SC neurons receive excitatory inputs from the retina (red) and from V1 (orange) and an inhibitory feed-back (grey) from interneurons located deeper in the SC. Superficial excitatory neurons contact deeper premotor neurons and neurons in the dLGN. Premotor neurons in the deep layer feed gaze centers of the brain. Adapted from [
11].
1.2. Cortical and Subcortical Plasticity
Activity-dependent plasticity in the visual system was classically thought to be exclusively expressed at the cortical level [
22], whereas subcortical areas such as the dLGN and the SC were traditionally considered to be involved in transmission of visual signals and thus expressing much less to no plasticity. Supporting this idea, monocular deprivation has been thought for a long time to produce no change in receptive field properties of dLGN neurons, dating back to the pioneering work by Wiesel and Hubel in 1963 [
23,
24,
25,
26]. Thus, for many years, the dogma was that functional plasticity occurs only in the superior visual areas located in the cerebral cortex where visual information is processed and possibly stored, whereas subcortical areas are only devoted to a rigid processing of visual information. However, this dichotomous view between noble and subaltern visual areas has been challenged by later works indicating that subcortical areas do express functional plasticity and actively participate in both the elaboration of perceptual decision-making and cognitive functions [
3,
27].
2. Functional Plasticity in Subcortical Visual Areas
2.1. Functional Plasticity in the dLGN
The notion that dLGN neurons do not express plasticity was disproved a decade after Wiesel and Hubel’s publication. Indeed, Ikeda and Wright showed in 1976 that the spatial resolution of dLGN neurons activated by the deviating eye in kittens reared with a squint is considerably reduced compared to that of neurons activated by the normal eye [28]. This result was the first to suggest that loss of normal binocular vision leads to plastic changes in the LGN. Later on, it was shown in amblyopic patients that functional deficits in visual response are already observed at the stage of the dLGN [29]. In addition, in contrast to what was previously assumed, about half of the rodent dLGN relay neurons in a given monocular territory in fact receive inputs from both eyes, indicating a potential binocularity for a large proportion of dLGN neurons [30,31,32,33,34,35,36]. Moreover, spatial receptive fields at eye opening in mouse dLGN are ~2 times larger than in adulthood due to an increase in surround suppression owing to an increased in feed-forward inhibition [37]. Furthermore, monocular deprivation (MD) in the mouse has been shown to produce a large shift in ocular dominance (OD) in dLGN neurons (Figure 2A) [38,39,40]. In one of these studies, GABAergic synaptic inhibition was found to be critical [39]. It is very unlikely that the plasticity observed in the dLGN only represents altered feedback from the cortex, because the shift in dLGN responses was resistant to cortical inactivation using the GABAA receptor agonist muscimol [38].
2.2. Functional Plasticity in the SC
The SC is the mammalian equivalent of the optic tectum in inferior vertebrates [5]. While many studies reported functional and synaptic plasticity in the tadpole optic tectum [43,44,45,46], fewer investigations have been performed on the mammalian SC. As for the dLGN, the SC was thought to be largely devoid of functional plasticity, since receptive field features were found unchanged after MD [47]. However, recent findings suggest that SC express functional plasticity. The best demonstration of SC plasticity comes from studies on hemianopia, a permanent visual deficit caused by cortical trauma [41,42,48,49]. Patients with unilateral hemianopia are totally blind in the contralateral visual hemi-field but have preserved subcortical visual structures such as the SC. In basic post-traumatic conditions, hemianopia patients display a total lack of gaze orientation towards the blind hemi-field; a behavioral response depending on cortico-collicular connections. However, when the visual stimulus was temporally paired with an auditory stimulus occurring in the same region of the visual space (i.e., audio-visual training), normal gaze orientation towards the blind hemi-field (Figure 2B) was observed both in patients [41] and cats [42,49]. Interestingly, the re-emergence of visual behavior in cats is correlated with the reinstatement of visual responsiveness in deep layer neurons of the ipsilesional SC [42]. This audio-visual training procedure is thought to be related to the Hebbian learning and to reflect potentiation of visually activated synapses onto gaze-orientation related premotor neurons within the SC that fired under the conjoint activation of auditory synapses. In fact, audio-visual training has been shown to be able to reveal auditory or visual responses that were absent initially [50].
3. Structural Plasticity in Subcortical Visual Areas
3.1. Structural Plasticity in the dLGN
The visual system is immature at birth and several structural plasticity phenomena occur during early post-natal development, but also at later ages. In particular, a profound reorganization occurs at the retino-geniculate synapse during early post-natal development. For instance, the number of retinal ganglion cells innervating a relay neuron of mouse dLGN decreases from about 10 before eye opening to 1 at the adult stage [
57,
58] (
Figure 3A). This refinement occurs mainly by means of synapse elimination, synapse strengthening and clustering of synaptic boutons [
59]. In addition, the dendritic tree in both relay neurons and interneurons evolves during the first weeks of post-natal development, from small to large arborization with a transient peak in dendritic complexity at the time of eye opening [
60,
61].
3.2. Structural Plasticity in the SC
Structural plasticity has also been reported in the SC during post-natal development. Retino-collicular synapses in adult rodents follow the retino-topic organization [
75]. Early studies have shown that this topographic organization is preserved upon partial lesions of the SC, thanks to an orderly compression of the entire retinal projections onto the remaining SC [
76]. Conversely, following partial retinal lesion, remaining axons connect their correct targets to maintain the retinotopic map [
77]. These studies suggest that complex signaling mechanisms exist to dynamically establish the topographic organization of retino-collicular connections. However, during early development, retinal axons transiently branch and arborize in inappropriate regions of the SC [
78], resulting in topographically diffuse retinal projections. Thus, the ordered projection found in adults ultimately emerges after competitive interactions between retino-collicular contacts.
4. Synaptic Plasticity in Subcortical Visual Areas
4.1. Synaptic Plasticity in the dLGN
4.1.1. Hebbian Synaptic Plasticity in the dLGN
Hebbian synaptic plasticity at the retino-thalamic synapse was first demonstrated in 2007 by the group of Carla Shatz [
62]. Patterned spontaneous activity in the developing retina is known to drive synaptic refinement in the dLGN before eye opening [
81,
82,
83,
84]. Using burst-based activity patterns mimicking retinal waves, Shatz and colleagues showed that, before eye opening, synaptic plasticity at retinogeniculate synapse depends on the relative timing between pre- and post-synaptic bursts [
62]. Following the Hebbian principle reported at hippocampal synapses [
85,
86], coincident bursts produced long-term synaptic potentiation (LTP), whereas non-overlapping bursts produced mild synaptic long-term depression (LTD) [
62] (
Figure 3B). Such LTP induced by pre- and post-synaptic synchronous bursts is likely to be involved in the stabilization of the winner synapses during synaptic refinement. On the other hand, LTD induced by asynchronous burst is likely to reflect preliminary steps leading to synapse elimination [
87]. Supporting this idea, deletion of two proteins from the major histocompatibility complex class I (MHC I) required in CNS development and plasticity [
88,
89,
90,
91,
92] not only suppresses synapse elimination, but also eliminates LTD [
93].
4.1.2. Homeostatic Synaptic Plasticity in the dLGN
Homeostatic regulation of synaptic transmission has been reported in dLGN neurons following MD in mice [
63]. Interestingly, homeostatic regulation occurs on cortico-thalamic inputs but not on retino-thalamic inputs, suggesting that MD introduces a complex redistribution of synaptic weight in dLGN relay neurons (
Figure 3C). In this case, presynaptic release probability was found to be higher at the cortical input on the deprived side [
63]. However, no change on the post-synaptic side has been reported.
4.2. Synaptic Plasticity in the SC
4.2.1. Hebbian Synaptic Plasticity in the SC
While many studies reported long-lasting Hebbian synaptic plasticity in the tadpole optic tectum [
43,
94,
95], fewer investigations were performed on long-term synaptic plasticity in the mammalian SC. The first evidence for LTP induction in the SC was provided 30 years ago in the superficial layer of the SC following electrical stimulation at 50 Hz of the optic tract [
96]. Since then, several studies have been devoted to identifying the involved cellular mechanisms [
97,
98]. Interestingly, eye opening itself induces synaptic potentiation in the SC similar to NMDAR-dependent LTP at retino-collicular synapse [
99]. Moreover, the cellular mechanisms triggered by eye opening are the prerequisites for the induction of further LTP in the developing rat SC [
100]. However, no investigation has been undertaken, so far, to check whether the excitatory synapse linking visual neurons from the upper layer of the SC to premotor neurons from deeper layer express Hebbian potentiation, as suggested by behavioral studies [
41,
42,
48].
LTD has been also reported in the SC. Remarkably, LTD is specifically induced at strong but not weak inputs [
101], thus suggesting that cooperativity among many activated synapses is required for LTD induction in this structure.
4.2.2. Homeostatic Synaptic Plasticity in the SC
Homeostatic synaptic plasticity has been reported in the optic tectum of the tadpole following chronic visual deprivation [
102] or mechanosensory stimulation [
103]. However, no data about homeostatic plasticity have been reported so far in the SC.
5. Intrinsic Plasticity in Subcortical Visual Areas
Beyond synaptic plasticity, changes in intrinsic neuronal excitability represent the other side of functional plasticity that usually goes hand-in-hand with synaptic modifications [104,105] and possibly participate to developmental plasticity and learning [106,107]. Intrinsic plasticity is generally triggered by synaptic activity (induction phase) that induces plasticity of synaptic transmission in parallel. However, the expression of plasticity of intrinsic neuronal excitability depends on the regulation of voltage-gated ion channels (expression phase) such as hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [108,109,110,111], Nav [112], Kv1 [113] and Kv7 [114] channels.
6. Conclusions
The analysis of the current knowledge on activity-dependent plasticity leads to important conclusions. First, the role of dLGN neurons is not limited to a simple relay linking the retina to the cortex but dLGN neurons are capable of complex integration of visual signals arising from the retina, the cortex and the SC. dLGN neurons also participate to cognitive functions and do express functional and synaptic plasticity. Similarly, SC neurons also display cognitive functions, express functional and synaptic plasticity and have recently been shown to be involved in the recovery from hemianopia, in working memory and in saccade adaptation.
Many questions remain unsolved. First, the molecular mechanisms underlying functional plasticity are underexplored, and further studies will be required to decipher the key molecular actors involved in functional plasticity in subcortical visual areas. Second, while synaptic plasticity at excitatory synapses have been characterized in subcortical visual areas, little is known about inhibitory synaptic plasticity [
150]. Third, the plasticity of intrinsic neuronal excitability has not been explored in detail in the dLGN and the SC. There is no doubt that many of these questions will be addressed in the future.
This entry is adapted from the peer-reviewed paper 10.3390/cells10113162