COVID-19 is caused by a coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The difficulty in containing SARS-CoV-2 has underscored the need for techniques such as mass spectrometry in the diagnosis and treatment of COVID-19. Mass spectrometry-based methods have been employed in several studies to detect changes in interactions among host proteins, and between host and viral proteins in COVID-19 patients. The methods have also been used to characterize host and viral proteins, and analyze lipid metabolism in COVID-19 patients. Information obtained using the above methods are complemented by high-throughput analysis of transcriptomic and epigenomic changes associated with COVID-19, coupled with next-generation sequencing.
1. Proteomics and Mass Spectrometry in Coronavirus Disease 2019 (COVID-19)
MS-based proteomics is currently being used to identify SARS-CoV-2 infections and develop treatments for COVID-19. Liquid chromatography paired with tandem mass spectrometry (LC-MS/MS) is a technique that incorporates both the separation capabilities of liquid chromatography and the comprehensive analysis acquired by combining multiple mass spectrometers [
51,
52,
53]. It is a method being utilized to positively identify the presence of SARS-CoV-2 in human samples. This method has been able to discern the SARS-CoV-2 nucleoprotein NCAP_SARS2 from 1500 distinguished proteins in COVID-19 positive nasopharyngeal samples (which were already determined as positive through quantitative reverse transcription PCR) [
54]. Compared to methods such as RT-PCR and rapid tests, LC-MS/MS has shown both high sensitivity and specificity while also decreasing the risk of false-positives [
41,
55]. These findings support the use of MS-based proteomics in the diagnosis of COVID-19.
1.1. COVID-19-Linked Host Protein Characterization Discovered through Mass Spectrometric Proteomics
Along with the initial diagnosis of COVID-19, MS-based proteomic methods have been utilized to determine specific biomarkers of severe COVID-19 cases. A study by Shen et al. utilized Tandem Mass Tag pro, a chemical tag used for the identification of proteins in samples [
56], coupled with ultra performance LC-MS/MS (a highly sensitive type of LC-MS/MS) to identify the abnormal regulation of several apolipoproteins in COVID-19 positive blood serum samples [
30]. Several of these lipoproteins, namely APOA-I (Apolipoprotein A1) and APOM (Apolipoprotein M), had implications in severe COVID-19 cases.
1.2. Proteomics in COVID-19 Treatment Identification
Several treatments for COVID-19 have been identified through MS-based proteomics; the development of these treatments relies on the identification of SARS-CoV-2 replication machinery.
A study by Gordon et al. pursued ligands of SARS-CoV-2 interacting proteins in an attempt to distinguish targets for treatment [
1]. After the discovery of 69 such compounds, further testing was performed to name two successful treatment classes: inhibitors of protein synthesis (such as ternatin-4 and zotatifin) and ligands of Sigma receptors (such as hydroxychloroquine and haloperidol). Both classes were observed to have effects on SARS-CoV-2 replication, identified through a decrease in the viral nucleoprotein over an 8 h timespan. The prior identification of virus-host protein interactions through AP-MS was an integral step in the discovery of the two treatment classes [
1].
In addition to host–virus protein interactions, the discovery of case severity related protein biomarkers has also contributed to the identification of COVID-19 treatments. By distinguishing 38 proteins with differing levels of expression in mild versus severe cases of COVID-19, utilizing label-free quantitative MS, Suvarna et al. identified disruptions in several cellular processes. These processes include blood coagulation, for which proteins such as alpha-2-macroglobulin and fibrinogen gamma chain are upregulated in severe COVID-19 cases, and inflammation, for which proteins such as Serpin Family A Member 3 are upregulated in severe COVID-19 cases. Nine of the 38 identified proteins were then utilized to test the efficacy of COVID-19 treatments, resulting in additional supporting evidence for previously identified treatments such as Selinexor (an inhibitor of viral replication) [
31].
2. Lipidomics and Mass Spectrometry in COVID-19
2.1. The Immunomodulatory Axis of COVID-19 Disease Burden
During an active COVID-19 infection with lung presentation, a burst of hyperinflammation involving the production of pro-inflammatory secreted glycoproteins (cytokines) exacerbates the immune reaction and leads to loss of cellular integrity leading to a profound enhancement in immune-mediated responses. This cytokine eruption in the infected lung epithelia and endothelia is the product of cellular membrane VLCPUFAs transformation to eicosanoids which promote the migration and activation of macrophages and granulocytes including basophils and neutrophils [
80]. High levels of the growth factor, macrophage-stimulating colony factor (MSCF) will promote myeloid differentiation to macrophages along with costimulatory IL-3 and IL-6. The granulocyte lineage are differentiated from the myeloid precursor population via GSCF along with the same pro-inflammatory cytokines [
81].
Type 1 macrophages (M1) are pro-inflammatory and induce virally infected cellular death while type 2 macrophages (M2) inhibit this process while secondarily functioning to remove cellular debris via the phagocytic functions of this subgroup. PPARα transcriptionally regulates the production of fatty acid beta oxidation genes. M1 pro-inflammatory macrophages work through the TLR 2 receptor to promote glycolysis over fatty acid β-oxidation and thus remain polarized as potent pro-inflammatory macrophages [
82].
COVID-19 transmission and infection kinetics appear to map onto a biphasic progression wherein there is an asymptomatic phase that may end the disease progression or, if virion titre is sufficient and co-morbidities obtain, there can be a second phase where frank respiratory disease presents with a sizable increase in cytokine and chemokine expression and secretion followed by a hyperimmune response [
88].
Depending on the progression curve of the disease presentation, the inappropriate introduction of pharmacotherapeutic corticosteroids to COVID-19 patients, prior to massive cytokine and chemokine expression, can actually increase disease severity and enhance the possibility of poor prognosis and the ultimate outcome axis [
86]. It has been shown that classical epithelial immune initiation starts with the production of pro-inflammatory mediators including the eicosanoids, LTB4 and PG2 which enhance inflammation until the “eicosanoid switch”, which marks the end of initiation; this is when the relative cellular concentrations of PGE2 plus PGD2 are equal to LTB4 [
52].
At this point, resolution is apprehended by the production of anti-inflammatory eicosanoids, the resolvins and PGF2a. Pathogenic induction of endocrine stress hormones such as norepinephrine trigger subsequent cortisol resistance such that the “eicosanoid switch” is invoked when the molar concentration of norepinephrine balances out the concentrations of glucose-sensing serum insulin plus that of cortisol, after which, corticosteroid efficacy is restored. When norepinephrine levels drop to the non-stimulated nadir, steady-state corticosteroid tonicity is restored [
52]. Therefore, the alteration of cortisol efficacy with the inflammatory signaling axis recommends that the pharmacological introduction of corticosteroids during an active infection, of COVID-19, could corrupt this balance leading to a hyper-inflammatory response and poor disease outcome.
2.3. Mass Spectrometry-Based Studies on Spike Protein Helps in Vaccine Development
Techniques based on mass spectrometry have been critical for understanding the glycosylation of SARS-CoV-2 spike protein [
27,
101,
102,
103,
104,
105,
106]. Glycosylation of the spike protein of coronavirus and other viral proteins are crucial to the infection process because the modification shields immunogenic epitopes, and impact immunological pressure across the protein surface [
107,
108]. In this regard, the entire signature of carbohydrates in a cell or organism is called glycome, which is under immense focus in SARS-CoV-2 research [
109,
110,
111,
112]. The studies have contributed towards the design and development of some of the vaccines against COVID-19 [
113,
114,
115,
116].
2.4. Signaling Pattern-Recognition Receptors (PRRs) Are Found on Multiple Host-Cell Surfaces
A series of signaling pattern-recognition receptors known as toll-like receptors (TLRs) are found on the surface of a variety of defense cells and other cells. These TLRs play a major role in the induction of innate immunity and contribute to the induction of adaptive immunity.
The binding of a microbial PAMP to its intracellular immune or epithelial cell TLR (or other PRR) transmits a signal to the host cell’s nucleus inducing the expression of genes coding for the synthesis of intracellular regulatory molecules including cytokines [
117]. The cytokines, in turn, bind to cytokine receptors on other defense cells. Unique combinations of TLRs appear in different cell types and may occur in pairs. Different TLRs directly or indirectly bind different microbial surface molecules. These signaling PRRs, which are found in the membranes of the endosomes (phagolysosomes) are used to enhance the intracellular degredatioin of the pathogens.
2.5. Sphingolipids at the Center of COVID-19 Infection Dynamics
There is good reason to assume the protein transmembrane domains, typically composed of hydrophobic amino acid side chains, induce their insertion into the membrane via higher order lipid domain transformations from helix type II to bilayer transitions as coordinated by surface tension molecular regulation [
126].
In a recent review of sphingolipids and SARS-CoV-2, it was established that lung cell ceramide facilitated the infection process, while sphingosine-1P inhibited it [
20].
This implicates the sphingomyelinase biosynthetic route since the neutral SMAse isoform of the enzyme is located in the plasma membrane and upon activation would generate in situ CER from membrane sphingomyelin thus promoting this metabolic route for viral entry instead of de novo synthesis from palmitoyl CoA plus L-serine or the salvage routes through sphingosine phosphate or glycosphingolipid pathways.
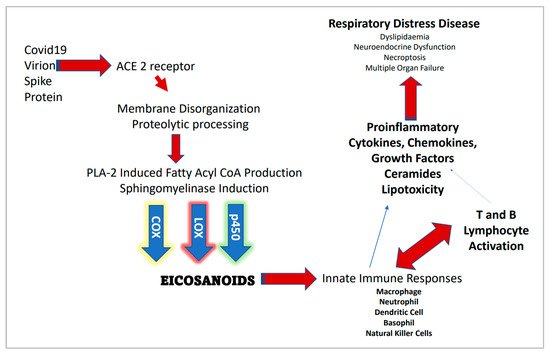
However, acid SMase (aSMase) inhibitors such as amitriptyline and pharmacotherapeutics such as hydroxychloroquine that raise the pH of the phagolysosome (thus inhibiting aSMase) may suggest intracellular CER synthesis and thus membrane lipid raft assembly is also involved in the decrease of COVID-19 transmission and disease severity. Figure 1 provides a general outline for the proteomic/lipidomic axis in COVID-19 transmittance and pathology.
Figure 1. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and associated proteomic/lipidomic axis.
2.6. Contributions of Lipidomics in Understanding and Treating COVID-19
Overall, lipidomics studies reveal several important phenomenon associated with COVID-19 as outlined below. The viral replication and life-cycle is critically impacted by host lipids serving as double-membrane vesicles and other roles. Viral entry and propagation are regulated by lipid biogenesis [
33]. Lipidomic analysis of COVID-19 patients in conjunction with quantification of proinflammatory cytokines and alarmins show a correlation between patient lipid profiles and factors like IL-26, TSLP (thymic stromal lymphopoietin) and adiponectin [
33].
Lipidomic and metabolomic profiles showed common signatures despite heterogeneous clinical symptoms of COVID-19 patients, where treatment with the immunosuppressive drug tocilizumab partially reversed the metabolic changes caused by COVID-19 [
40]. Dislipidemia, i.e., lipid imbalance with pathological consequences, is reported in a study which observed changes in levels of ceramides [
35], increased triglycerides and decreased cholesteryl esters [
34]. A study on COVID-19 acute phase patients reported severe dyslipidemia which impacts size and distribution of lipoprotein particles, dysregulation of central metabolism, accumulation of ketone bodies, and upregulation of succinate similar to the oncogenic pseudohypoxic environment [
38].
This entry is adapted from the peer-reviewed paper 10.3390/biochem1030016