G. mellonella, also known as a wax moth, belongs to Lepidoptera order from the Pyralidae family. This moth is distributed worldwide, and is commercially available for fishing or to feed reptiles and birds, making them readily accessible. The last larval stage of this insect has been utilized as a host model to extensively study bacteria and fungi pathogenesis, including Acinetobacter baumannii
- host–pathogen interactions
- virulence factors
- therapy strategies
1. Introduction
Over the past decades, Acinetobacter baumannii has widely emerged as one of the major causes of highly invasive nosocomial pathogen infections in the health system [1]. Infections by this microorganism are responsible for increased morbidity and mortality, and make a huge burden to patients and hospitals [2]. As the top concerning microorganism on the global priority list ranked by the World Health Organization (WHO) [3], A. baumannii is a multi-drug resistant (MDR) bacterium which needs new drug development [4]. Therefore, the screening of the most adapted animal models for studying pathogenic mechanisms and therapeutic strategies before clinical therapies is particularly critical.
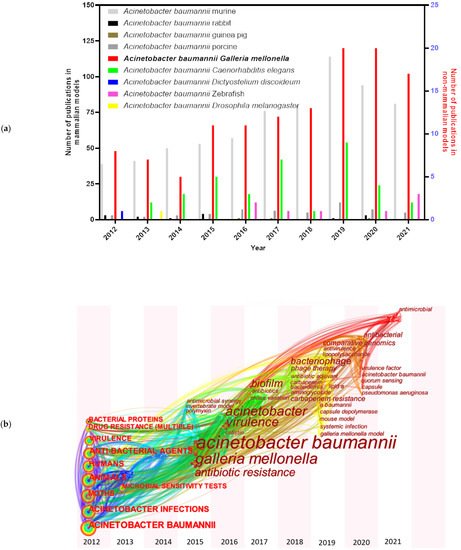
A series of animal models have been examined and established for A. baumannii studies, including mammalian and non-mammalian models. Murine models [5] are still the predominant mammalian models in A. baumannii researches, though some other mammalian models have also been tested, such as rabbits [6], guinea pigs [7], and porcine models [8] ( Figure 1 a). A. baumannii is frequently associated with pneumonia, making small rodent lung infection models well suited for these bacteria [9]. However, increasing costs and growing ethical concerns made the use of rodents more difficult [10]. Non-mammalian models, such as Galleria mellonella (greater wax moth) [11], Caenorhabditis elegans (roundworm) [12], Dictyostelium discoideum (slime mold) [13], Danio rerio (zebrafish) [14] and Drosophila melanogaster (common fruit fly) [9], are also informative to decipher virulence factors needed during host–pathogen interactions of A. baumannii . Among them, G. mellonella caterpillars have attracted more and more attention in the last ten years ( Figure 1 a). The keywords for each node distributed in time-zone visualization ( Figure 1 b) indicate an increased interest towards the G. mellonella model system. The research involving G. mellonella model mainly focused on A. baumannii pathogenicity factors (such as surface antigen proteins and efflux pump) and drug therapies.
The benefits of using G. mellonella models are numerous. G. mellonella produce a huge progeny quantity with a short life cycle, and are inexpensive because they are easy to rear without special laboratory infrastructure. The possibility of using many animals per experiment makes them eligible for high-throughput studies. The relatively large size of the larvae (12–20 mm) allows precise quantification of the inoculation, and facilitates handling for tissue extraction and histological analysis [28,29]. Importantly, there is no ethical approval requirement for research on G. mellonella [30].
Despite a large number of articles describing the feasibility and safety of G. mellonella for microbial studies [31], its value for drug-resistant microorganisms remains to be explored. In this review, we highlight why G. mellonella can be used as a model for MDR A. baumannii infection, the contributions of this model to study A. baumannii pathogenicity, and to target the most effective and prospective therapy strategies to fight A. baumannii infection.
2. G. mellonella-Based Model
G. mellonella has a rapid life cycle with four developmental stages: egg; larvae; pupa; and adult moth [32] ( Figure 2 a). Differences in temperature and humidity affect the developmental speed, with a full life cycle under favorable conditions being only 8–12 weeks [33]. The white dome-shaped eggs hatch to larvae in about 1–2 weeks at 28–34 °C [33]. The creamy-colored larvae pass through 8–10 molting stages in 5–6 weeks until cocoon development [33]. After 2–3 weeks of incubation, the reddish-brown pupa evolves into a pale cream moth [33].
Insects’ innate immune system has been well documented to protect them against infection from a broad spectrum of pathogens [34]. Genome research has shown that larvae have many homologous genes to humans, who participate in pathogen recognition and signal transduction [35]. In G. mellonella , the innate immune system is constituted by cuticle, cellular, and humoral immune defense [36].
The cellular immune system is mediated by phagocytic cells, called hemocytes, which are mainly responsible of encapsulation, nodulation, and phagocytosis [30,37] ( Figure 2 b). To date, six out of the eight types of hemocytes found in insects have been identified to be responsible of these functions in G. mellonella (plasmatocytes, granulocytes, prohemocytes, spherulocytes, coagulocytes, and oenocytoids) [28,38]. Firstly, granular cells attack the penetrated microorganisms, then, the process promotes the attachment of plasmatocytes to form a layer of cells, resulting in encapsulation and nodulation. Phagocytosis is similar to human cellular defense reactions with the participation of hemocytes [31]. The humoral immune response is highly regulated by soluble effectors, such as complement-like proteins (opsonins), melanin, and antimicrobial peptides (AMPs), which play a role in melanization, hemolymph clotting, and primary immunization [39] ( Figure 2 c).
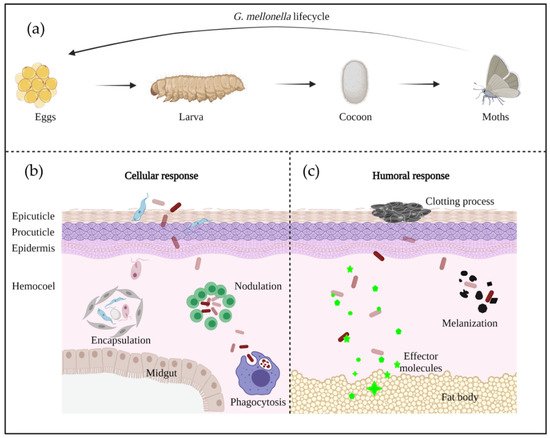
In the early stage of A. baumannii invasion, the larval immune response is activated, and struggles against A. baumannii virulence factors. If the infection is controlled by the immune system, the larvae will survive—alternatively, the larvae will continue melanization and finally die. The two different responses are dependent of the phagocytosis by hemocytes, or the melanization caused by the deposition of melanin around microorganisms [40].
3. Experimental Design Suitable for G. mellonella/A. baumannii Interaction
Generally, the larvae are employed at the 5th to 6th instar, at about 2–3 cm length and a weight of around 250–350 mg. The spontaneous mobility of larvae is a good indication of their viability [33,41]. For one experiment, the larvae are conventionally divided into three groups of about 10 to 20 individuals, one group inoculated with PBS, one group with bacteria sub-divided by the different conditions/strains needed, and one group without injection. In Table 1 and Table 2 , the inoculation methods, culture conditions, and larval detection indicators are listed. These different studies have described virulence factors of A. baumannii ( Table 1 ) and antimicrobial agents tested against A. baumannii ( Table 2 ) in G. mellonella .
| A. baumannii | Larva/ Group |
Larva Inoculation | Larva Incubation | Refs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pathogenicity | Strains and Mutants | Style | Volume/Larva | Concentration | Temp | Time | |||
| Virulence factors | |||||||||
| Phospholipases | Phospholipases C | ΔplcN | 20 | Injection | 10 µL | 2 × 106 CFU/mL | 37 °C | 8 days | [42,43] |
| ATCC 19606T, plc2::aph, plc1::aph-FRT, plc1::ermAM/plc2::aph | 10 | Injection | 1 × 105 CFU | 37 °C | 5 days | ||||
| Phospholipases D | ATCC 19606T, Δpld | 16 | Injection | 10 µL | 1 × 106 CFU/mL | 37 °C | 4 days | [44] | |
| Membrane proteins | Surface antigen protein 1 (SurA1) | ATCC 17978, CCGGD201101, ΔSurA1 | 20 | Injection | 20 µL | 1 × 106 CFU/mL | 37 °C | 7 days | [45] |
| Capsular polysaccharides and LOS | Capsule genes, epsA and ptk | AB5075, AB5075 epsA::Tn5, AB5075 ptk::Tn5 | - | Injection | 5 µL | 1 × 107 CFU/mL | 37 °C | 5 days | [46] |
| K locus | MDR-ZJ06, ΔgnaA | - | Injection | 10 µL | 1 × 108 CFU/mL | 37 °C | 3 days | [47] | |
| ptk gene | AB5075, Δptk | - | Injection | - | 1 × 105, 1 × 106 CFU | 37 °C | 6 days | [48] | |
| LOS | ATCC 17978, ΔlpxO, ΔlpxO::Tn7lpxO | 10 | Injection | 10 µL | 5 × 104 CFU | 37 °C | 3 days | [49] | |
| Protein secretion system | Type VI secretion system (T6SS) | DSM30011, ΔtssM | 20 | Injection | 10 µL | 1 × 105 CFU | 37 °C | - | [50,51] |
| 17978, 17978 ΔtssM | 10 | Injection | 5 µL | 106–107 CFU | 37 °C | 40–60 h | |||
| Metal acquisition systems | Iron acquisition | ATCC 19606T, basD, bauA | 30, 10 | Injection | 5 µL | 1 × 102, 1 × 105 CFU | 37 °C | 18 h/6 days | [52,53,54,55] |
| A118, ATCC 19606T, ATCC 17978 | - | Injection | - | 1 × 105 CFU | 37 °C | 6 days | |||
| ATCC 19606T, ΔbasD | 30 | Injection | 10 µL | OD600: 0.2 | 37 °C | 72 h | |||
| ATCC 19606T, entA::aph, tonB1::aph, tonB2::aacC1, tonB1::aph tonB2::aacC1 | 10 | Injection | - | 1 × 105 CFU | 37 °C | 6 days | |||
| Zinc acquisition | AB5075, znuB::Tn | - | Injection | - | 1 × 106 CFU | 37 °C | 0 h, 4 h | [48] | |
| Antimicrobial resistance | |||||||||
| β-lactamases | AB5075, ZJ06, LS01, ATCC 17978 | 10 | Injection | 10 µL | OD600: 0.1 | 37 °C | 72 h | [56] | |
| Efflux pumps | ATCC 17978, A1S | 16 | Injection | 10 µL | OD600: 0.5 | 37 °C | 96 h | [57] | |
| Permeability defects | ATCC 19606, ΔkupΔtrkΔkdp, ΔkupΔtrk | 20 | Injection | 10 µL | 1 × 106 CFU | 37 °C | 6 days | [58] | |
| Aminoglycoside modifying enzymes | AbA155 | 10 | Injection | 5 µL | 5 × 105 CFU | 37 °C | >120 h | [59] | |
| Alternation of target sites | MB_2, MB_6C, MB_23C, MB_177, MB_90, MB_119, SG3161, SG3166 | 10 | Injection | - | 1 × 105 CFU | 37 °C | 96 h | [60] | |
| Dissemination | |||||||||
| Quorum sensing | 3-hydroxy-C12-homoserine lactone | M2, aba1::Km | 16 | Injection | 10 µL | >0.5 log CFU | 37 °C | 6 days | [40] |
| abaM gene | AB5075, abaI::T26, abaM::T26 | 10 | Injection | - | 2 × 104 CFU, 2 × 105 CFU | 37 °C | 120 h | [61] | |
| Biofilm | NCTC 12156, NCTC 10303, ATCC 17978, NCTC 13302, W1, NCTC 13423, ATCC BAA-1710, NCTC 13424, ATCC BAA-1709, UKA1-UKA19 | 10 | Injection | - | 1 × 105, 1 × 106 CFU | 37 °C | 5 days | [62] | |
| Motility | ATCC 17978, 129/ddc, 277/dat | 16 | Injection | 5 µL | 3 × 105 CFU | 37 °C | 5 days | [63] | |
| Others | |||||||||
| Stress response | Reactive oxygen species (ROS) resistance | ATCC 17978, ATCC 17978 sod2343::Km, ATCC 17978 sod2343::Km pWHsod2343 | 16, 10 | Injection | 5 µL | 3 × 105 CFU, 1.5 × 106 CFU | 37 °C, −80 °C | 5 days, immediately | [64] |
| Temperature | ATCC 17978 | - | Injection | 10 µL | 1 × 106 CFU/mL | 28 °C, 37 °C | 72 h | [65] | |
| Ethanol | ATCC 19606T | 30 | Injection | - | 1 × 105 CFU | 37 °C | 6 days | [66] | |
| Phase-variable switch | AB5075 opaque, AB5075 translucent | 10 | Injection | - | 3 × 104 CFU | 37 °C | 24 h | [67,68] | |
| AB5075, ΔompR, ΔenvZ, ΔompR ΔenvZ | 30 | Injection | - | 103–104 CFU | 37 °C | 5 days | |||
| Category | A. baumannii | Treatment Type | Dose | Time | Refs | |
|---|---|---|---|---|---|---|
| Volume/Larva | Concentration | |||||
| AMPs | ||||||
| Amphiphilic peptide zp3 | - | Post-treatment | 10 µL | 200–800 mg/kg | 30 min | [69] |
| Anti-lpxB pPNA | MDR | Post-treatment | 10 µL | 75 mg/kg | 1 h | [70] |
| PNA (RXR)4 XB | MDR | Post-treatment | 10 µL | 150/600 µM | 30 min | [71] |
| Antibiotics | ||||||
| Colistin | MDR | Post-treatment | 10 µL | 2.5 mg/kg | 30 min | [70,72,73,74,75,76] |
| - | Post-treatment | 10 µL | 2.5 mg/kg | 30 min | ||
| Clinical isolate | Post-treatment | 10 µL | 2.5 mg/kg | 2 h | ||
| Carbapenem-resistant | Post-treatment | 10 µL | 2.5 mg/kg | 2 h | ||
| Colistin-resistant | Post-treatment | 5 µL | 2.5 mg/kg | 30 ± 5 min | ||
| MDR | Post-treatment | 10 µL | 40 mg/kg | - | ||
| MDR | Post-treatment | 10 µL | 2 mg/kg | 1 h | ||
| Cefozopran | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Ciprofloxacin | - | Post-treatment | - | 10 mg/kg | 20 min | [77] |
| Clarithromycin | MDR | Pre-treatment | 5 µL | 25 mg/kg | 2.5 h | [78] |
| Cotrimoxazole | Carbapenem-resistant | Post-treatment | 10 µL | 10 mg/kg | 2 h | [75] |
| Doripenem | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg | 30 ± 5 min | [79] |
| Gentamicin | - | Post-treatment | - | 8 mg/kg | 20 min | [72,77] |
| - | Post-treatment | - | 8 mg/kg | 20 min | ||
| Imipenem | MDR | Post-treatment | - | 5 mg/mL | 30 min | [80] |
| Levofloxacin | MDR | Post-treatment | 10 µL | 6.7 mg/kg | 2 h | [74] |
| Meropenem | Clinical isolate | Post-treatment | 10 µL | 4 mg/kg | 1 h | [77,81] |
| - | Post-treatment | - | 20 mg/kg | 20 min | ||
| Minocycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Mitomycin C | - | Post-treatment | - | 13–16 mg/kg | 2–5 min | [82] |
| Netropsin | Clinical isolate | Post-treatment | 5 µL | 12.5 mg/L | 30 min | [83] |
| Novobiocin | MDR | Post-treatment | 10 µL | 100 mg/kg | 3 h | [84] |
| Polymyxin B | Clinical isolate | Post-treatment | 5 µL | 4 mg/L | 30 min | [76,83] |
| MDR | Post-treatment | 10 µL | 40 mg/kg | - | ||
| Rifampicin | MDR | Post-treatment | 2 µL | 2.5, 5, 10 mg/kg | 30 min | [85] |
| Sitafloxacin | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Teicoplanin | MDR | Post-treatment | 10 µL | 10 mg/kg | 30 min | [72] |
| Telavancin | - | Post-treatment | 10 µL | 10 mg/kg | 30 min | [73] |
| Tetracycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Tigecycline | MDR | Post-treatment | 10 µL | 40 mg/kg | - | [76] |
| Vancomycin | Colistin-resistant | Post-treatment | 5 µL | 15 mg/kg | 30 ± 5 min | [79] |
| Cotrimoxazole/colistin | Carbapenem-resistant | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 2 h | [75] |
| Daptomycin/colistin | MDR | Post-treatment | - | 4 mg/L + 2.5 mg/L | 2 h | [86] |
| Doripenem/Vancomycin | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg + 15 mg/kg | 30 ± 5 min | [79] |
| Doripenem/Vancomycin/colistin | Colistin-resistant | Post-treatment | 5 µL | 7.5 mg/kg + 15 mg/kg + 2.5 mg/kg | 30 ± 5 min | [79] |
| Levofloxacin/colistin | MDR | Post-treatment | 10 µL | 6.7 mg/kg + 2.5 mg/kg | 2 h | [74] |
| Polymyxin B/netropsin | Clinical isolate | Post-treatment | 5 µL | 4 mg/L + 12.5 mg/L | 30 min | [83] |
| Teicoplanin/colistin | MDR | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | [72] |
| Telavancin/colistin | - | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | [73] |
| Vancomycin/colistin | MDR | Post-treatment | 10 µL | 15 mg/kg + 2.5 mg/kg | 2 h | [72,87] |
| MDR | Post-treatment | 10 µL | 10 mg/kg + 2.5 mg/kg | 30 min | ||
| Others | ||||||
| Anti-lpxB pPNA/colistin | MDR | Post-treatment | 10 µL | 75 mg/kg + 2 mg/kg | 1 h | [70] |
| Bacteriophage | Carbapenem-resistant | Post-treatment | 5 µL | 1 × 1010, 1 × 109 PFU/mL | 30 min | [11,77,80,88] |
| - | Post-treatment | - | MOI ≈ 1 | 20 min | ||
| MDR | Post-treatment | 10 µL | 5.107 PFU, MOI = 100 | 30 min | ||
| Carbapenem-resistant | Post-treatment | 10 µL | 104 pfu | 30 min | ||
| Capsule depolymerase Dpo48 | Extensive drug-resistant | Pre-treatment, post-treatment | 10 µL | 50 µg/mL, 5 µg | 1 h, 5 min | [89] |
| Epicatechin | MDR | Post-treatment | - | 40 mg/kg | 30 min | [90] |
| Homodimeric Tobramycin Adjuvant/Novobiocin | MDR | Post-treatment | 10 µL | 25/50 mg/kg + 25/50 mg/kg | 3 h | [84] |
| Gallium nitrate | MDR | Post-treatment | - | 1.2 mmol/kg | 15 min | [91] |
| Gallium protoporphyrin IX | - | Simultaneously | 5 µL | 20, 40 µg/mL | - | [92] |
| Manganese (i) tricarbonyl complexes | MDR | Post-treatment | - | 5 mg/kg | 30 min | [93] |
| SCH-79797 | MDR | Simultaneously | 66.6 µg/larva | - | [94] | |
| Silver acetate | Carbapenem-resistant | Post-treatment | - | 0, 10, 20 mg/kg | 30 min | [95] |
| Theaflavin | MDR | Post-treatment | - | 20 mg/kg | 30 min | [90] |
| Theaflavin/Epicatechin | MDR | Post-treatment | - | 20 mg/kg + 40 mg/kg | 30 min | [90] |
| Bacteriophage/Ciprofloxacin | - | Post-treatment | - | MOI ≈ 1 + 10 mg/kg | 20 min | [77] |
| Bacteriophage/Gentamicin | - | Post-treatment | - | MOI ≈ 1 + 8 mg/kg | 20 min | [77] |
| Bacteriophage/Meropenem | - | Post-treatment | - | MOI ≈ 1 + 20 mg/kg | 20 min | [77] |
| Endolysin/colistin | - | Post-treatment | 10 µL | 25 µg/mL + 1/4 MIC | 1 h | [96] |
Notes: MDR: multi-drug resistant. Pre-treatment/post-treatment: the antimicrobial agents were added before/after the A. baumannii infection. MOI: multiplicity of infection. CFU: colony forming unit. Time: the period between the first and second injection.
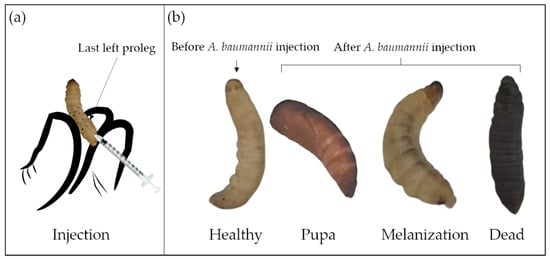
After 24 h of starvation at room temperature, three inoculation methods have been described to work with G. mellonella : topical application [97]; force-feeding [98]; and injection [11]. For A. baumannii infection, only the injection method into the hemocoel of the larval cuticle of the last left proleg [40] has been used ( Figure 3 a). For drug treatment, the correct timing of drug administration is also important, commonly within 3 h after A. baumannii injection. In some studies, drug application before or simultaneously with A. baumannii infection has been reported, but such cases are rare [89,91,94]. Compared to the two other methods, the injection has the advantage to accurately deliver the inoculum, and is therefore more reproducible [40]. However, the control group, injected only with buffer or medium, is crucial to ensure that the death of larvae is not caused by trauma or solvents.
G. mellonella larvae can be maintained at different temperatures after injection, between 15 °C to over 37 °C [99]. In order to better understand the interaction between the host and the pathogen in an environment closer to the mammalian organism, 37 °C is the most employed temperature for A. baumannii infection [29]. The viability, motility, and virulence of A. baumannii at 28 °C [65] and 30 °C [40] were also studied in order to assess the adaptability of the different clinical strains’ response to environmental changes. The incubation duration inside the larvae usually varies from few hours to few days. Experiments suggest that too short periods (<4 h) are not conducive to an accurate evaluation of A. baumannii virulence or drug efficacy. Conversely, after too long (>8 days) time periods, the larvae metamorphose into moths.
The G. mellonella larvae assessments could be larval mobility [90], mortality/survival rate [72], histological analysis [11], and bacterial numbers recovered after incubation [64]. Table 3 introduces the health index scoring system to evaluate the larval health status, including larvae mobility, cocoon formation, melanization, and survival [99]. The movement, observed by touching and the melanization, visible by naked eyes, are keys to distinguish the larval morbidity after A. baumannii infection ( Figure 3 b) [90]. Though the A. baumannii virulence overcomes the larval immune system over time, the larval movement gradually decreases, and the melanization progresses gradually. Complete melanization indicates death. Mortality/survival rate is the most monitored indicator, which directly reflects A. baumannii virulence. The survival percentage, usually characterized by the Kaplan–Meier curve, is investigated every 24 h [100]. Histological analyses are essential for studying host–defense mechanisms and pathogen infection pathways. A rare study associated with tissue damage, fat body, and muscle layer melanization has been reported for A. baumannii infected larvae [11].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens10111483
